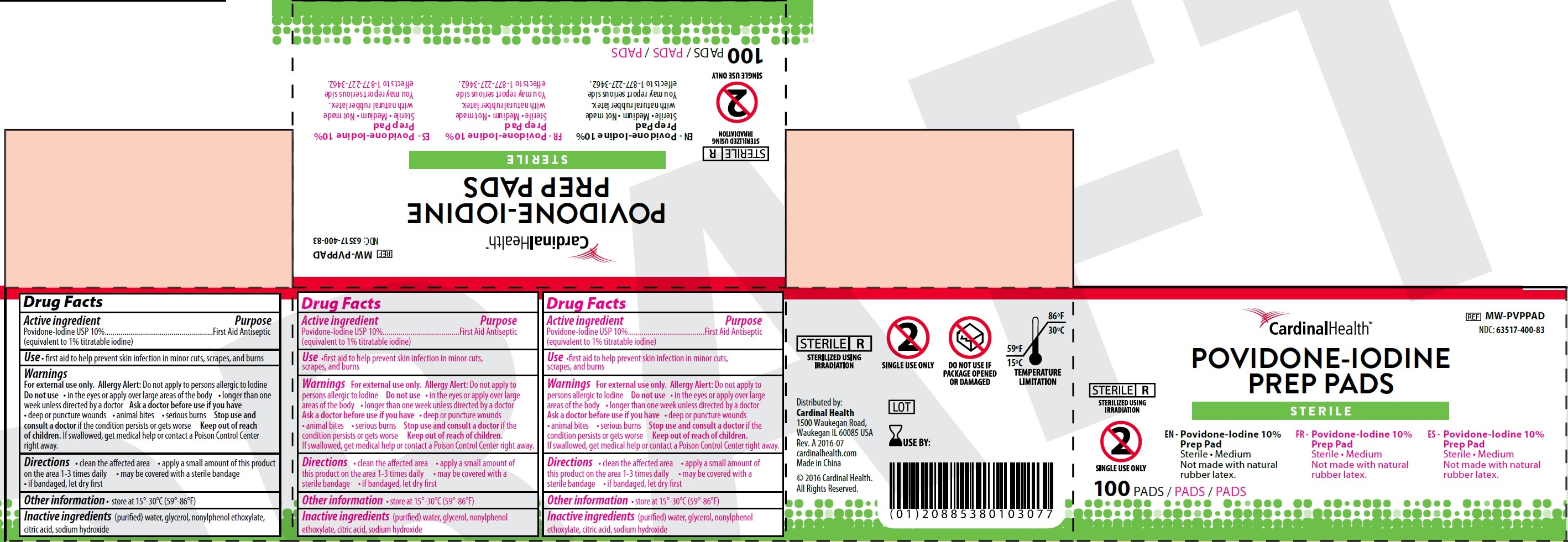
Active ingredient
Povidone-Iodine USP 10% (equivalent to 1% titratable iodine)
Purpose
First Aid Antiseptic
Use
•first aid to help prevent skin infection in minor cuts, scrapes, and burns
Warnings
For external use only. Do not apply to persons allergic to Iodine
Allergy Alert:
Do not use
• in the eyes or apply over large areas of the body • longer than one week unless directed by a doctor
Ask a doctor before use if you have
• deep or puncture wounds • animal bites • serious burns
Stop use and consult a doctor
if the condition persists or gets worse
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• clean the affected area • apply a small amount of this product on the area 1-3 times daily • may be covered with a sterile bandage • if bandaged, let dry first
Other information
• store at 15°-30°C (59°-86°F)
Inactive ingredients
(purified) water, glycerol, nonylphenol ethoxylate, citric acid, sodium hydroxide
Package Labeling:
Packet Labeling:

Box Labeling:
Carton Labeling:
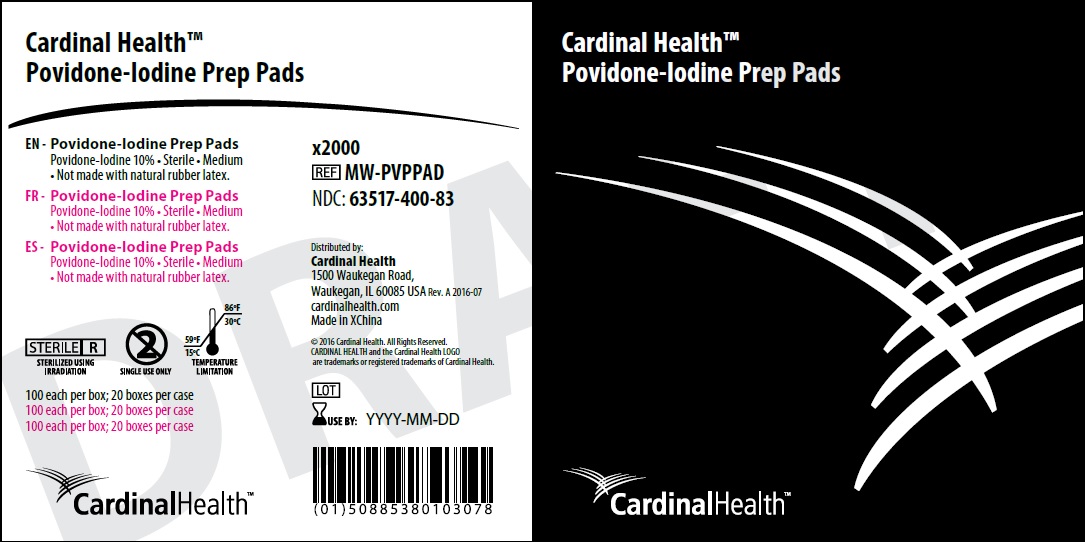
Cardinal Health 200, Inc.


