DESCRIPTION
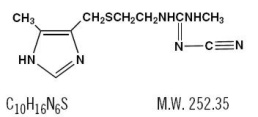
Cimetidine is a histamine H2-receptor antagonist. Chemically it is N"-cyano-N-methyl-N'-[2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]-ethyl]guanidine. Its structural formula is:
Cimetidine contains an imidazole ring, and is chemically related to histamine.
Cimetidine has a bitter taste and characteristic odor.
Solubility Characteristics
Cimetidine is soluble in alcohol, slightly soluble in water, very slightly soluble in chloroform and insoluble in ether.
Each tablet, for oral administration, contains 200 mg, 300 mg, 400 mg or 800 mg cimetidine, USP. Inactive ingredients are: croscarmellose sodium, crospovidone, hypromellose, lecithin, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, pregelatinized starch (corn), sodium alginate, sodium lauryl sulfate, titanium dioxide, triacetin, vanillin, FD&C Blue No. 1 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake and D&C Yellow No. 10 Aluminum Lake.
CLINICAL PHARMACOLOGY
Cimetidine tablets competitively inhibits the action of histamine at the histamine H2 receptors of the parietal cells and thus is a histamine H2-receptor antagonist.
Cimetidine is not an anticholinergic agent. Studies have shown that cimetidine tablets inhibit both daytime and nocturnal basal gastric acid secretion. Cimetidine tablets also inhibit gastric acid secretion stimulated by food, histamine, pentagastrin, caffeine and insulin.
Antisecretory Activity
1) Acid Secretion
Nocturnal
An 800 mg oral dose of cimetidine tablets at bedtime reduces mean hourly H+ activity by greater than 85% over an 8-hour period in duodenal ulcer patients, with no effect on daytime acid secretion. A 1,600 mg oral dose of cimetidine tablets at bedtime produces 100% inhibition of mean hourly H+ activity over an 8-hour period in duodenal ulcer patients, but also reduces H+ activity by 35% for an additional 5 hours into the following morning. Cimetidine tablets given as 400 mg twice daily and 300 mg 4 times daily decreases nocturnal acid secretion in a dose-related manner, i.e., 47% to 83% over a 6- to 8-hour period and 54% over a 9-hour period, respectively.
Food Stimulated
During the first hour after a standard experimental meal, a 300 mg oral dose of cimetidine tablets inhibited gastric acid secretion in duodenal ulcer patients by at least 50%. During the subsequent 2 hours cimetidine tablets inhibited gastric acid secretion by at least 75%.
The effect of a 300 mg breakfast dose of cimetidine tablets continued for at least 4 hours and there was partial suppression of the rise in gastric acid secretion following the luncheon meal in duodenal ulcer patients. This suppression of gastric acid output was enhanced and could be maintained by another 300 mg dose of cimetidine tablets given with lunch.
In another study, a 300 mg dose of cimetidine tablets given with the meal increased gastric pH as compared with placebo.
|
Cimetidine |
Placebo |
|
|
1 hour |
3.5 |
2.6 |
|
2 hours |
3.1 |
1.6 |
|
3 hours |
3.8 |
1.9 |
|
4 hours |
6.1 |
2.2 |
24-Hour Mean H+ Activity
Cimetidine tablets dosed at 800 mg at bedtime, 400 mg twice daily, and 300 mg 4 times daily, all provide a similar, moderate (less than 60%) level of 24-hour acid suppression. However, the 800 mg bedtime dose regimen exerts its entire effect on nocturnal acid, and does not affect daytime gastric physiology.
Chemically Stimulated
Cimetidine tablets administered orally significantly inhibited gastric acid secretion stimulated by betazole (an isomer of histamine), pentagastrin, caffeine and insulin as follows:
|
Stimulant |
Stimulant Dose |
Cimetidine Tablets |
% Inhibition |
|
Betazole |
1.5 mg/kg (sc) |
300 mg (po) |
85% at 2 1/2 hours |
|
Pentagastrin |
6 mcg/kg/hr (iv) |
100 mg/hr (iv) |
60% at 1 hour |
|
Caffeine |
5 mg/kg/hr (iv) |
300 mg (po) |
100% at 1 hour |
|
Insulin |
0.03 units/kg/hr (iv) |
100 mg/hr (iv) |
82% at 1 hour |
When food and betazole were used to stimulate secretion, inhibition of hydrogen ion concentration usually ranged from 45% to 75% and the inhibition of volume ranged from 30% to 65%.
Pharmacokinetics
Cimetidine tablets are rapidly absorbed after oral administration and peak levels occur in 45 to 90 minutes. The half-life of cimetidine tablets is approximately 2 hours. Blood concentrations remain above that required to provide 80% inhibition of basal gastric acid secretion for 4 to 5 hours following a dose of 300 mg.
Following parenteral administration, most of the drug is excreted as the parent compound in the urine, the principle route of excretion of cimetidine tablets. After oral administration, the drug is extensively metabolized in which the sulfoxide is the major metabolite. Following a single oral dose, 48% of the drug is recovered from the urine after 24 hours as the parent compound.
CLINICAL TRIALS
Duodenal Ulcer
Cimetidine tablets have been shown to be effective in the treatment of active duodenal ulcer and, at reduced dosage, in maintenance therapy following healing of active ulcers.
Active Duodenal Ulcer
Cimetidine tablets accelerate the rate of duodenal ulcer healing. Healing rates reported in U.S. and foreign controlled trials with cimetidine tablets are summarized below, beginning with the regimen providing the lowest nocturnal dose.
|
||||
|
Regimen |
300 mg 4 times daily |
400 mg twice daily |
800 mg at bedtime |
1600 mg at bedtime |
|
Week 4 |
68% |
73% |
80% |
86% |
|
Week 6 |
80% |
80% |
89% |
- |
|
Week 8 |
- |
92% |
94% |
- |
A U.S., double-blind, placebo-controlled, dose-ranging study demonstrated that all once-daily at bedtime regimens of cimetidine tablets were superior to placebo in ulcer healing and that 800 mg of cimetidine tablets at bedtime healed 75% of patients at 4 weeks. The healing rate with 800 mg at bedtime was significantly superior to 400 mg at bedtime (66%) and not significantly different from 1600 mg at bedtime (81%).
In the U.S. dose-ranging trial, over 80% of patients receiving 800 mg of cimetidine tablets at bedtime experienced nocturnal pain relief after one day. Relief from daytime pain was reported in approximately 70% of patients after 2 days. As with ulcer healing, the 800 mg dose at bedtime was superior to 400 mg at bedtime and not different from 1,600 mg at bedtime.
In foreign, double-blind studies with 800 mg of cimetidine tablets at bedtime, 79% to 85% of patients were healed at 4 weeks.
While short-term treatment with cimetidine tablets can result in complete healing of the duodenal ulcer, acute therapy will not prevent ulcer recurrence after cimetidine tablets have been discontinued. Some follow-up studies have reported that the rate of recurrence once therapy was discontinued was slightly higher for patients healed on cimetidine tablets than for patients healed on other forms of therapy; however, the patients treated with cimetidine tablets generally had more severe disease.
Maintenance Therapy in Duodenal Ulcer
Treatment with a reduced dose of cimetidine tablets have been proven effective as maintenance therapy following healing of active duodenal ulcers.
In numerous placebo-controlled studies conducted worldwide, the percent of patients with observed ulcers at the end of 1 year’s therapy with 400 mg of cimetidine tablets at bedtime was significantly lower (10% to 45%) than in patients receiving placebo (44% to 70%). Thus, from 55% to 90% of patients were maintained free of observed ulcers at the end of 1 year with 400 mg of cimetidine tablets at bedtime.
Factors such as smoking, duration and severity of disease, gender, and genetic traits may contribute to variations in actual percentages.
Trials of other anti-ulcer therapy, whether placebo-controlled, positive-controlled or open, have demonstrated a range of results similar to that seen with cimetidine tablets.
Active Benign Gastric Ulcer
Cimetidine tablets have been shown to be effective in the short-term treatment of active benign gastric ulcer.
In a multicenter, double-blind U.S. study, patients with endoscopically confirmed benign gastric ulcer were treated with 300 mg of cimetidine tablets 4 times a day or with placebo for 6 weeks. Patients were limited to those with ulcers ranging from 0.5 to 2.5 cm in size. Endoscopically confirmed healing at 6 weeks was seen in significantly* more patients treated with cimetidine tablets than in patients receiving placebo, as shown below:
|
||
|
Cimetidine Tablets (300 mg, 4 times daily) |
Placebo |
|
|
Week 2 |
14/63 (22%) |
7/63 (11%) |
|
Total at week 6 |
43/65 (66%)* |
30/67 (45%) |
In a similar multicenter U.S. study of the 800 mg bedtime oral regimen, the endoscopically confirmed healing rates were:
|
||
|
Cimetidine Tablets (800 mg at bedtime) |
Placebo |
|
|
Total at week 6 |
63/83 (76%)* |
44/80 (55%) |
Similarly, in worldwide double-blind clinical studies, endoscopically evaluated benign gastric ulcer healing rates were consistently higher with cimetidine tablets than with placebo.
Gastroesophageal Reflux Disease
In 2 multicenter, double-blind, placebo-controlled studies in patients with gastroesophageal reflux disease (GERD) and endoscopically proven erosions and/or ulcers, cimetidine tablets were significantly more effective than placebo in healing lesions. The endoscopically confirmed healing rates were:
|
Trial |
Cimetidine Tablets (800 mg twice daily) |
Cimetidine Tablets (400 mg 4 times daily) |
Placebo |
p-Value (800 mg twice daily vs. placebo) |
|
|
1 |
Week 6 |
45% |
52% |
26% |
0.02 |
|
Week 12 |
60% |
66% |
42% |
0.02 |
|
|
2 |
Week 6 |
50% |
20% |
<0.01 |
|
|
Week 12 |
67% |
36% |
<0.01 |
In these trials cimetidine tablets were superior to placebo by most measures in improving symptoms of day- and night-time heartburn, with many of the differences statistically significant. The 4 times-daily regimen was generally somewhat better than the twice-daily regimen where these were compared.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison Syndrome):
Cimetidine tablets significantly inhibited gastric acid secretion and reduced occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger-Ellison Syndrome, systemic mastocytosis, and multiple endocrine adenomas. Use of cimetidine tablets were also followed by healing of intractable ulcers.
INDICATIONS AND USAGE
Cimetidine tablets are indicated in:
1. Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks and there is rarely reason to use cimetidine tablets at full dosage for longer than 6 to 8 weeks (see DOSAGE AND ADMINISTRATION: Duodenal Ulcer). Concomitant antacids should be given as needed for relief of pain. However, simultaneous administration of cimetidine tablets and antacids is not recommended, since antacids have been reported to interfere with the absorption of cimetidine.
2. Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of active ulcer. Patients have been maintained on continued treatment with cimetidine tablets 400 mg at bedtime for periods of up to 5 years.
3. Short-term treatment of active benign gastric ulcer. There is no information concerning usefulness of treatment periods of longer than 8 weeks.
4. Erosive gastroesophageal reflux (GERD). Erosive esophagitis diagnosed by endoscopy. Treatment is indicated for 12 weeks for healing of lesions and control of symptoms. The use of cimetidine tablets beyond 12 weeks has not been established (see DOSAGE AND ADMINISTRATION: GERD).
5. The treatment of pathological hypersecretory conditions (i.e., Zollinger-Ellison Syndrome, systemic mastocytosis, multiple endocrine adenomas).
CONTRAINDICATIONS
Cimetidine tablets are contraindicated for patients known to have hypersensitivity to the product.
PRECAUTIONS
General
Rare instances of cardiac arrhythmias and hypotension have been reported following the rapid administration of cimetidine hydrochloride injection by intravenous bolus.
Symptomatic response to treatment with cimetidine tablets do not preclude the presence of a gastric malignancy. There have been rare reports of transient healing of gastric ulcers despite subsequently documented malignancy.
Reversible confusional states (see ADVERSE REACTIONS) have been observed on occasion, predominantly, but not exclusively, in severely ill patients. Advancing age (50 or more years) and preexisting liver and/or renal disease appear to be contributing factors. In some patients these confusional states have been mild and have not required discontinuation of cimetidine tablets. In cases where discontinuation was judged necessary, the condition usually cleared within 3 to 4 days of drug withdrawal.
Drug Interactions
Cimetidine tablets, apparently through an effect on certain microsomal enzyme systems, has been reported to reduce the hepatic metabolism of warfarin-type anticoagulants, phenytoin, propranolol, nifedipine, chlordiazepoxide, diazepam, certain tricyclic antidepressants, lidocaine, theophylline, and metronidazole, thereby delaying elimination and increasing blood levels of these drugs.
Clinically significant effects have been reported with the warfarin anticoagulants; therefore, close monitoring of prothrombin time is recommended, and adjustment of the anticoagulant dose may be necessary when cimetidine tablets are administered concomitantly. Interaction with phenytoin, lidocaine, and theophylline has also been reported to produce adverse clinical effects.
However, a crossover study in healthy subjects receiving either 300 mg 4 times daily or 800 mg at bedtime of cimetidine tablets concomitantly with a 300 mg twice-daily dose of theophylline extended-release tablets demonstrated less alteration in steady-state theophylline peak serum levels with the 800 mg at bedtime regimen, particularly in subjects aged 54 years and older. Data beyond 10 days are not available. (Note: All patients receiving theophylline should be monitored appropriately, regardless of concomitant drug therapy.)
Dosage of the drugs mentioned above and other similarly metabolized drugs, particularly those of low therapeutic ratio or in patients with renal and/or hepatic impairment, may require adjustment when starting or stopping the concomitant administration of cimetidine tablets to maintain optimum therapeutic blood levels.
Alteration of pH may affect absorption of certain drugs (e.g., ketoconazole). If these products are needed, they should be given at least 2 hours before cimetidine administration.
Additional clinical experience may reveal other drugs affected by the concomitant administration of cimetidine tablets.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month toxicity study conducted in rats, at dose levels of 150, 378 and 950 mg/kg/day (approximately 8 to 48 times the recommended human dose), there was a small increase in the incidence of benign Leydig cell tumors in each dose group; when the combined drug-treated groups and control groups were compared, this increase reached statistical significance. In a subsequent 24-month study, there were no differences between the rats receiving 150 mg/kg/day and the untreated controls. However, a statistically significant increase in benign Leydig cell tumor incidence was seen in the rats that received 378 and 950 mg/kg/day. These tumors were common in control groups as well as treated groups and the difference became apparent only in aged rats.
Cimetidine has demonstrated a weak antiandrogenic effect. In animal studies this was manifested as reduced prostate and seminal vesicle weights. However, there was no impairment of mating performance or fertility, nor any harm to the fetus in these animals at doses 8 to 48 times the full therapeutic dose of cimetidine tablets, as compared with controls. The cases of gynecomastia seen in patients treated for 1 month or longer may be related to this effect.
In human studies, cimetidine tablets have been shown to have no effect on spermatogenesis, sperm count, motility, morphology or in vitro fertilizing capacity.
Pregnancy
Teratogenic Effects. Pregnancy Category B
Reproduction studies have been performed in rats, rabbits and mice at doses up to 40 times the normal human dose and have revealed no evidence of impaired fertility or harm to the fetus due to cimetidine tablets. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Cimetidine is secreted in human milk and, as a general rule, nursing should not be undertaken while a patient is on a drug.
Pediatric Use
Clinical experience in children is limited. Therefore, therapy with cimetidine tablets cannot be recommended for children under 16, unless, in the judgement of the physician, anticipated benefits outweigh the potential risks. In very limited experience, doses of 20 to 40 mg/kg/day have been used.
ADVERSE REACTIONS
Adverse effects reported in patients taking cimetidine tablets are described as follows by body system. Incidence figures of 1 in 100 and greater are generally derived from controlled clinical studies.
CNS
Headaches, ranging from mild to severe, have been reported in 3.5% of 924 patients taking 1,600 mg/day, 2.1% of 2,225 patients taking 800 mg/day and 2.3% of 1,897 patients taking placebo. Dizziness and somnolence (usually mild) have been reported in approximately 1 in 100 patients on either 1,600 mg/day or 800 mg/day.
Reversible confusional states, e.g., mental confusion, agitation, psychosis, depression, anxiety, hallucinations, disorientation, have been reported predominantly, but not exclusively, in severely ill patients. They have usually developed within 2 to 3 days of initiation of treatment with cimetidine tablets and have cleared within 3 to 4 days of discontinuation of the drug.
Endocrine
Gynecomastia has been reported in patients treated for 1 month or longer. In patients being treated for pathological hypersecretory states, this occurred in about 4% of cases while in all others the incidence was 0.3% to 1% in various studies. No evidence of induced endocrine dysfunction was found, and the condition remained unchanged or returned toward normal with continuing treatment with cimetidine tablets.
Reversible impotence has been reported in patients with pathological hypersecretory disorders, e.g., Zollinger-Ellison Syndrome, receiving cimetidine tablets, particularly in high doses, for at least 12 months (range 12 to 79 months, mean 38 months). However, in large-scale surveillance studies at regular dosage, the incidence has not exceeded that commonly reported in the general population.
Hematologic
Decreased white blood cell counts in patients treated with cimetidine tablets (approximately 1 per 100,000 patients), including agranulocytosis (approximately 3 per million patients), have been reported, including a few reports of recurrence on rechallenge. Most of these reports were in patients who had serious concomitant illnesses and received drugs and/or treatment known to produce neutropenia. Thrombocytopenia (approximately 3 per million patients) and, very rarely, cases of pancytopenia or aplastic anemia have also been reported. As with some other H2-receptor antagonists, there have been extremely rare reports of immune hemolytic anemia.
Hepatobiliary
Dose-related increases in serum transaminase have been reported. In most cases they did not progress with continued therapy and returned to normal at the end of therapy. There have been rare reports of cholestatic or mixed cholestatic-hepatocellular effects. These were usually reversible. Because of the predominance of cholestatic features, severe parenchymal injury is considered highly unlikely. However, as in the occasional liver injury with other H2-receptor antagonists, in exceedingly rare circumstances fatal outcomes have been reported.
There has been reported a single case of biopsy-proven periportal hepatic fibrosis in a patient receiving cimetidine tablets.
Rare cases of pancreatitis, which cleared on withdrawal of the drug, have been reported.
Hypersensitivity
Rare cases of fever and allergic reactions including anaphylaxis and hypersensitivity vasculitis, which cleared on withdrawal of the drug, have been reported.
Renal
Small, possibly dose-related increases in plasma creatinine, presumably due to competition for renal tubular secretion, are not uncommon and do not signify deteriorating renal function. Rare cases of interstitial nephritis and urinary retention, which cleared on withdrawal of the drug, have been reported.
Cardiovascular
Rare cases of bradycardia, tachycardia and AV heart block have been reported with H2-receptor antagonists.
Musculoskeletal
There have been rare reports of reversible arthralgia and myalgia; exacerbation of joint symptoms in patients with preexisting arthritis has also been reported. Such symptoms have usually been alleviated by a reduction in the dosage of cimetidine tablets. Rare cases of polymyositis have been reported, but no causal relationship has been established.
Integumental
Mild rash and, very rarely, cases of severe generalized skin reactions including Stevens-Johnson syndrome, epidermal necrolysis, erythema multiforme, exfoliative dermatitis and generalized exfoliative erythroderma have been reported with H2-receptor antagonists. Reversible alopecia has been reported very rarely.
Immune Function
There have been extremely rare reports of strongyloidiasis hyperinfection in immunocompromised patients.
Respiratory
A large epidemiological study suggested an increased risk of developing pneumonia in current users of histamine-2-receptor antagonists (H2RAs) compared to patients who had stopped H2RA treatment, with an observed adjusted relative risk of 1.63 (95% CI, 1.07 to 2.48). However, a causal relationship between use of H2RAs and pneumonia has not been established.
OVERDOSAGE
Studies in animals indicate that toxic doses are associated with respiratory failure and tachycardia that may be controlled by assisted respiration and the administration of a beta-blocker.
Reported acute ingestions orally of up to 20 grams have been associated with transient adverse effects similar to those encountered in normal clinical experience. The usual measures to remove unabsorbed material from the gastrointestinal tract, clinical monitoring, and supportive therapy should be employed.
There have been reports of severe CNS symptoms, including unresponsiveness, following ingestion of between 20 and 40 grams of cimetidine, and extremely rare reports following concomitant use of multiple CNS-active medications and ingestion of cimetidine at doses less than 20 grams. An elderly, terminally ill dehydrated patient with organic brain syndrome receiving concomitant antipsychotic agents and 4,800 mg of cimetidine intravenously over a 24-hour period experienced mental deterioration with reversal on discontinuation of cimetidine.
There have been two deaths in adults who were reported to have ingested over 40 grams orally on a single occasion.
DOSAGE AND ADMINISTRATION
Duodenal Ulcer
Active Duodenal Ulcer
Clinical studies have indicated that suppression of nocturnal acid is the most important factor in duodenal ulcer healing (see CLINICAL PHARMACOLOGY: Antisecretory Activity: Acid Secretion). This is supported by recent clinical trials (see CLINICAL TRIALS: Duodenal Ulcer: Active Duodenal Ulcer). Therefore, there is no apparent rationale, except for familiarity with use, for treating with anything other than a once-daily at bedtime dosage regimen.
In a U.S. dose-ranging study of 400 mg at bedtime, 800 mg at bedtime and 1600 mg at bedtime, a continuous dose-response relationship for ulcer healing was demonstrated.
However, 800 mg at bedtime is the dose of choice for most patients, as it provides a high healing rate (the difference between 800 mg at bedtime and 1,600 mg at bedtime being small), maximal pain relief, a decreased potential for drug interactions (see PRECAUTIONS: Drug Interactions) and maximal patient convenience. Patients unhealed at 4 weeks, or those with persistent symptoms, have been shown to benefit from 2 to 4 weeks of continued therapy.
It has been shown that patients who both have an endoscopically demonstrated ulcer larger than 1.0 cm and are also heavy smokers (i.e., smoke 1 pack of cigarettes or more per day) are more difficult to heal. There is some evidence which suggests that more rapid healing can be achieved in this subpopulation with 1,600 mg of cimetidine tablets at bedtime. While early pain relief with either 800 mg at bedtime or 1,600 mg at bedtime is equivalent in all patients, 1,600 mg at bedtime provides an appropriate alternative when it is important to ensure healing within 4 weeks for this subpopulation. Alternatively, approximately 94% of all patients will also heal in 8 weeks with 800 mg of cimetidine tablets at bedtime.
Other regimens of cimetidine tablets in the United States which have been shown to be effective are: 300 mg 4 times daily, with meals and at bedtime, the original regimen with which U.S. physicians have the most experience, and 400 mg twice daily, in the morning and at bedtime (see CLINICAL TRIALS: Duodenal Ulcer: Active Duodenal Ulcer).
Concomitant antacids should be given as needed for relief of pain. However, simultaneous administration of cimetidine tablets and antacids is not recommended, since antacids have been reported to interfere with the absorption of cimetidine.
While healing with cimetidine tablets often occurs during the first week or two, treatment should be continued for 4 to 6 weeks unless healing has been demonstrated by endoscopic examination.
Active Benign Gastric Ulcer
The recommended adult oral dosage for short-term treatment of active benign gastric ulcer is 800 mg at bedtime, or 300 mg 4 times a day with meals and at bedtime. Controlled clinical studies were limited to 6 weeks of treatment (see CLINICAL TRIALS). A dose of 800 mg at bedtime is the preferred regimen for most patients based upon convenience and reduced potential for drug interactions. Symptomatic response to cimetidine tablets does not preclude the presence of a gastric malignancy. It is important to follow gastric ulcer patients to assure rapid progress to complete healing.
Erosive Gastroesophageal Reflux Disease (GERD)
The recommended adult oral dosage for the treatment of erosive esophagitis that has been diagnosed by endoscopy is 1,600 mg daily in divided doses (800 mg twice daily or 400 mg 4 times daily) for 12 weeks. The use of cimetidine tablets beyond 12 weeks has not been established.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison Syndrome)
Recommended adult oral dosage: 300 mg 4 times a day with meals and at bedtime. In some patients it may be necessary to administer higher doses more frequently. Doses should be adjusted to individual patient needs, but should not usually exceed 2,400 mg per day and should continue as long as clinically indicated.
Dosage Adjustment for Patients with Impaired Renal Function
Patients with severely impaired renal function have been treated with cimetidine tablets. However, such usage has been very limited. On the basis of this experience the recommended dosage is 300 mg every 12 hours orally. Should the patient’s condition require, the frequency of dosing may be increased to every 8 hours or even further with caution. In severe renal failure, accumulation may occur and the lowest frequency of dosing compatible with an adequate patient response should be used. When liver impairment is also present, further reductions in dosage may be necessary. Hemodialysis reduces the level of circulating cimetidine tablets. Ideally, the dosage schedule should be adjusted so that the timing of a scheduled dose coincides with the end of hemodialysis.
HOW SUPPLIED
Cimetidine Tablets, USP are available containing 200 mg, 300 mg, 400 mg or 800 mg of cimetidine, USP.
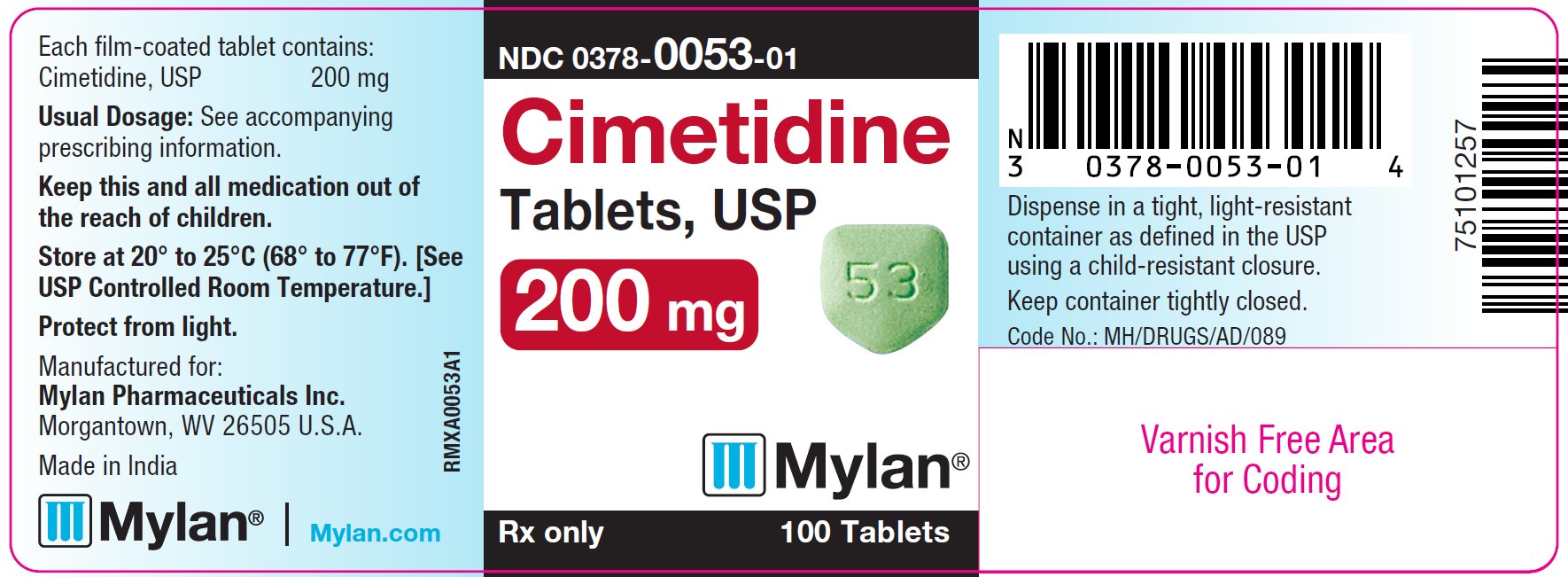
The 200 mg tablets are green, film-coated, five-sided, house-shaped, unscored tablets debossed with M on one side and 53 on the other side. They are available as follows:
NDC 0378-0053-01
bottles of 100 tablets
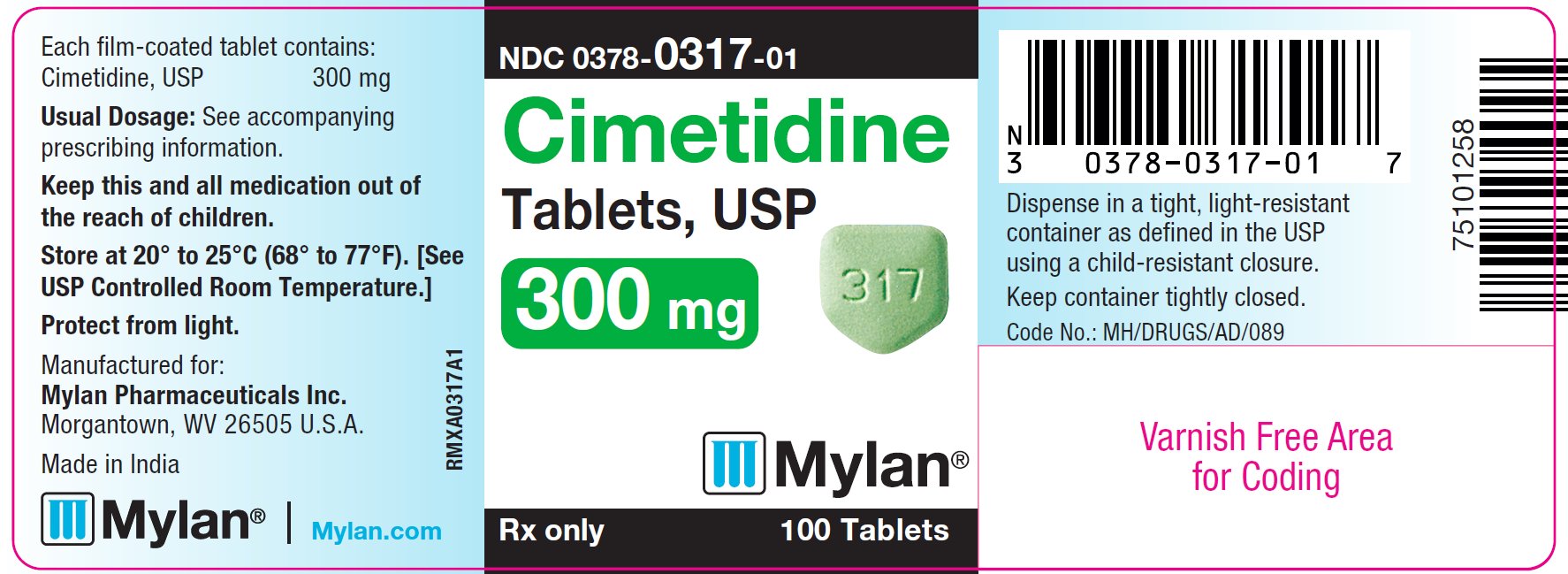
The 300 mg tablets are green, film-coated, five-sided, house-shaped, unscored tablets debossed with M on one side and 317 on the other side. They are available as follows:
NDC 0378-0317-01
bottles of 100 tablets
The 400 mg tablets are green, film-coated, five-sided, house-shaped, partially scored tablets debossed with M on one side and 372 on the other side. They are available as follows:
NDC 0378-0372-01
bottles of 100 tablets
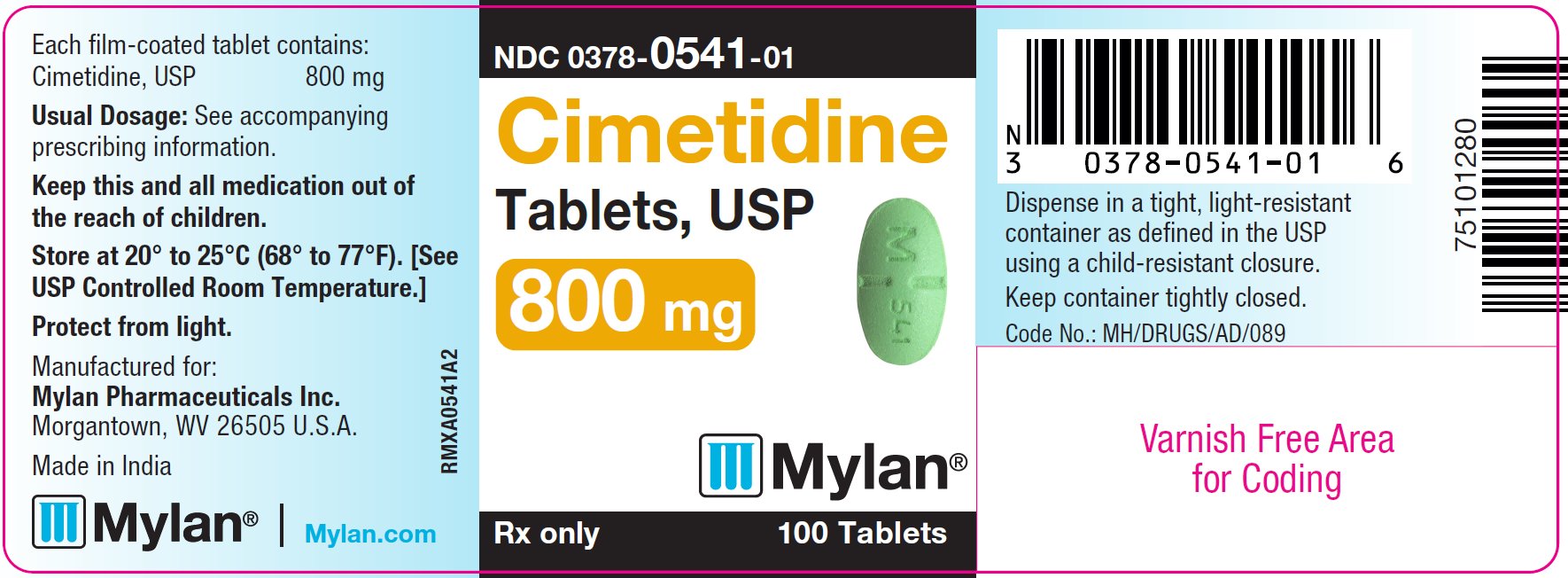
The 800 mg tablets are green, film-coated, oval, partially scored tablets debossed with M 541 across the partial score. They are available as follows:
NDC 0378-0541-01
bottles of 100 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Manufactured by:
Mylan Laboratories Limited
Hyderabad — 500 096, India
75069095
Revised: 8/2019
MXA:CIM:R1
PRINCIPAL DISPLAY PANEL - 200 mg
NDC 0378-0053-01
Cimetidine
Tablets, USP
200 mg
Rx only 100 Tablets
Each film-coated tablet contains:
Cimetidine, USP 200 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Protect from light.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0053A1
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089
PRINCIPAL DISPLAY PANEL - 300 mg
NDC 0378-0317-01
Cimetidine
Tablets, USP
300 mg
Rx only 100 Tablets
Each film-coated tablet contains:
Cimetidine, USP 300 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Protect from light.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0317A1
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089
PRINCIPAL DISPLAY PANEL - 400 mg
NDC 0378-0372-01
Cimetidine
Tablets, USP
400 mg
Rx only 100 Tablets
Each film-coated tablet contains:
Cimetidine, USP 400 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Protect from light.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0372A1
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089
PRINCIPAL DISPLAY PANEL - 800 mg
NDC 0378-0541-01
Cimetidine
Tablets, USP
800 mg
Rx only 100 Tablets
Each film-coated tablet contains:
Cimetidine, USP 800 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Protect from light.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0541A2
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089