OXYGEN CERTIFICATE OF ANALYSIS
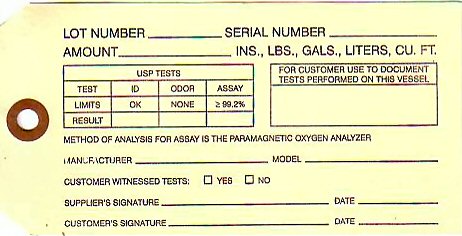
LOT NUMBER_____________ SERIAL NUMBER__________ AMOUNT__________INS, LBS., GALS., LITERS, CU. FT.
USP TESTS
TEST ID ODOR ASSAY
LIMITS OK NONE LESS THAN 99.2%
RESULT
FOR CUSTOMER TO USE TO DOCUMENT TESTS PERFORMED ON THIS VESSEL
METHOD OF ANALYSIS FOR ASSAY IS THE PARAMAGNETIC OXYGEN ANALYZER MANUFACTURER__________ MODEL______________ CUSTOMER WITNESSED TESTS YES NO SUPPLIERS SIGNATURE__________ DATE____________ CUSTOMERS SIGNATURE_______________ DATE________________
LIQUID MEDICAL GASES LIQUID OXYGEN DELIVERY TAG FILLED AND DISTRIBUTED BY____________ THIS VESSEL CONTAINS OXYGEN USP (SEE REVERSE SIDE FOR TEST RESULTS) OXYGEN PRODUCED BY THE AIR LIQUEFACTION PROCESS