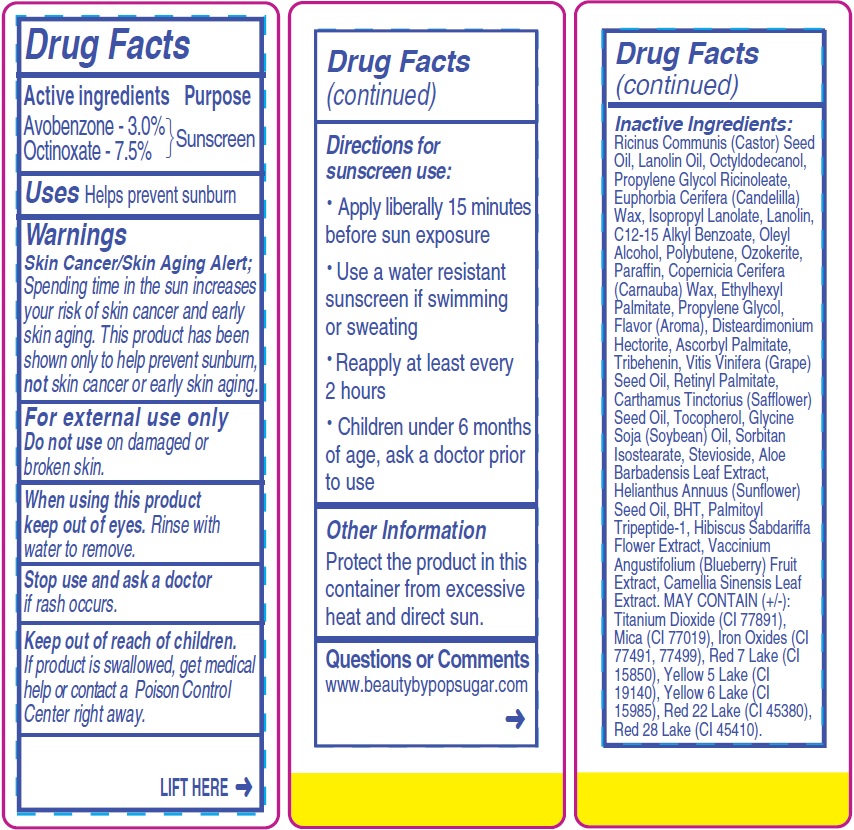
Warnings
Skin Cancer/Skin Aging Alert; Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Directions for sunscreen use:
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months of age, ask a doctor prior to use
Inactive Ingredients:
Ricinus Communis (Castor) Seed Oil, Lanolin Oil, Octyldodecanol, Propylene Glycol Ricinoleate, Euphorbia Cerifera (Candelilla) Wax, Isopropyl Lanolate, Lanolin, C12-15 Alkyl Benzoate, Oleyl Alcohol, Polybutene, Ozokerite, Paraffin, Copernicia Cerifera (Carnauba) Wax, Ethylhexyl Palmitate, Propylene Glycol, Flavor (Aroma), Disteardimonium Hectorite, Ascorbyl Palmitate, Tribehenin, Vitis Vinifera (Grape) Seed Oil, Retinyl Palmitate, Carthamus Tinctorius (Safflower) Seed Oil, Tocopherol, Glycine Soja (Soybean) Oil, Sorbitan Isostearate, Stevioside, Aloe Barbadensis Leaf Extract, Helianthus Annuus (Sunflower) Seed Oil, BHT, Palmitoyl Tripeptide-1, Hibiscus Sabdariffa Flower Extract, Vaccinium Angustifolium (Blueberry) Fruit Extract, Camellia Sinensis Leaf Extract. MAY CONTAIN (+/-): Titanium Dioxide (CI 77891), Mica (CI 77019), Iron Oxides (CI
77491, 77499), Red 7 Lake (CI 15850), Yellow 5 Lake (CI 19140), Yellow 6 Lake (CI 15985), Red 22 Lake (CI 45380), Red 28 Lake (CI 45410).