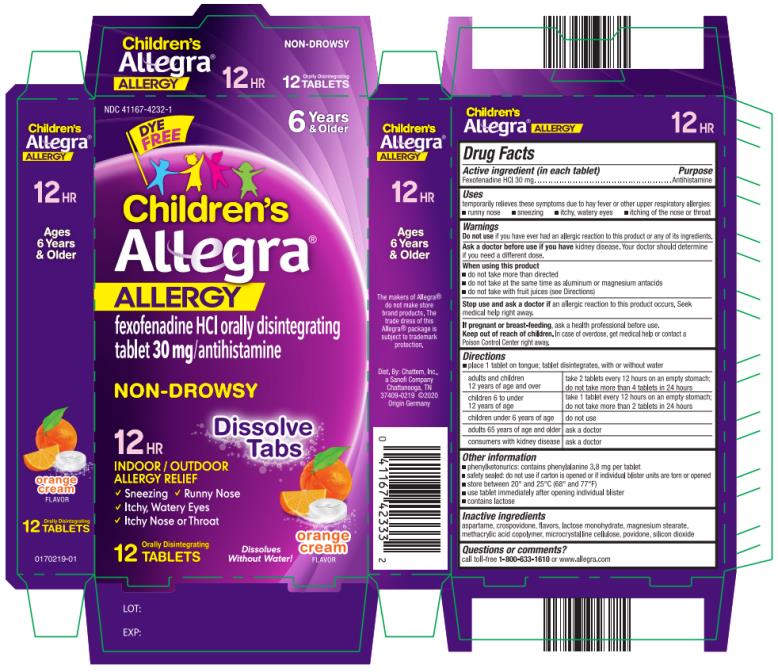
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, water eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Directions
- place 1 tablet on tongue; tablet disintegrates, with or without water
| adults and children 12 years of age and over | take 2 tablets every 12 hours on an empty stomach; do not take more than 4 tablets in 24 hours |
| children 6 to under 12 years of age | take 1 tablet every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours |
| children under 6 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
- phenylketonurics: contains phenylalanine 3.8 mg per tablet
- safety sealed: do not use if carton is opened or if individual blister units are torn or opened
- store between 20º and 25ºC (68º and 77ºF)
- use tablet immediately after opening individual blister
- contains lactose
Inactive ingredients
aspartame, crospovidone, flavors, lactose monohydrate, magnesium stearate, methacrylic acid copolymer, microcrystalline cellulose, povidone, silicon dioxide
Questions or comments?
call toll-free 1-800-633-1610 or www.allegra.com
Dist. By: Chattem, Inc, a Sanofi Company,
Chattanooga, TN 37409-0219 ©2015