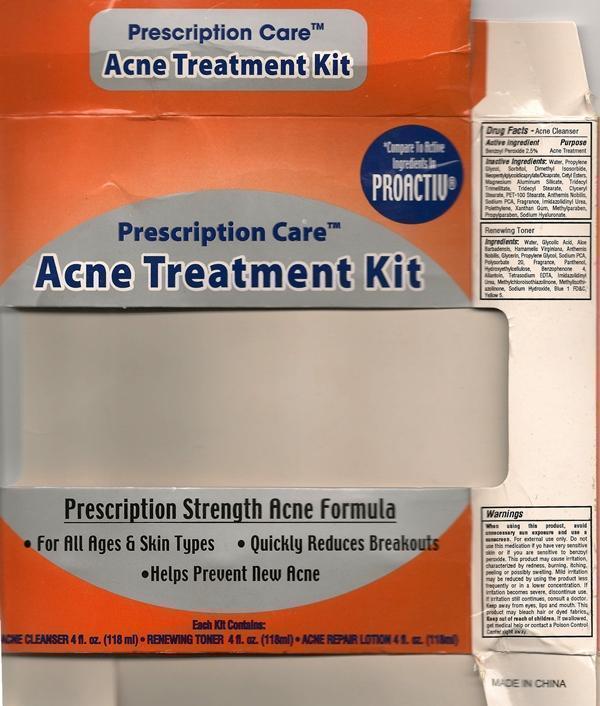
ACNE TREATMENT KIT PRESCRIPTION CARE- benzoyl peroxide
Blue Cross Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Acne Treatment Kit Repairing
Active Ingredients Purpose
Benzoyl Perioxide 2.5% Acne Treatment
Warnings
When using this product, avoid unnecessary sun exposure and use a sunscreen. Do not use this medication if you have very sensitive skin or if you are sensitive to benzoyl peroxide. This product may cause irritation, characterized by redness, burning, itching, peeling or possibly swelling. Mild irritation may be reduced by using the product less frequently or in a lower concentration. If irritation still continues, consult a doctor. Keep away from eyes, lips and mouth. This product may bleach hair or dyed fabrics. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredient
Inactive Ingredients: Water, Propylene Glycol, Ethoxydiglycol, Cetearyl Alcohol, Blyceryl Stearate, Ceteareth-20, Dimethicone, Panthenol, Fragrance, Cyclomethicone, Allantoin, Carbomer, Methylparaben, Propylparaben, Triethanol Amine, Diazolidinyl Urea, PEG-100, Stearate, Xanthan Gum
| ACNE TREATMENT KIT
PRESCRIPTION CARE
benzoyl peroxide kit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Blue Cross Laboratories (008298879) |
| Registrant - Blue Cross Laboratories (008298879) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ningbo Liyuan Daily Chemical Products Co., Ltd. | 530766098 | manufacture(22431-112, 22431-113, 22431-114) | |