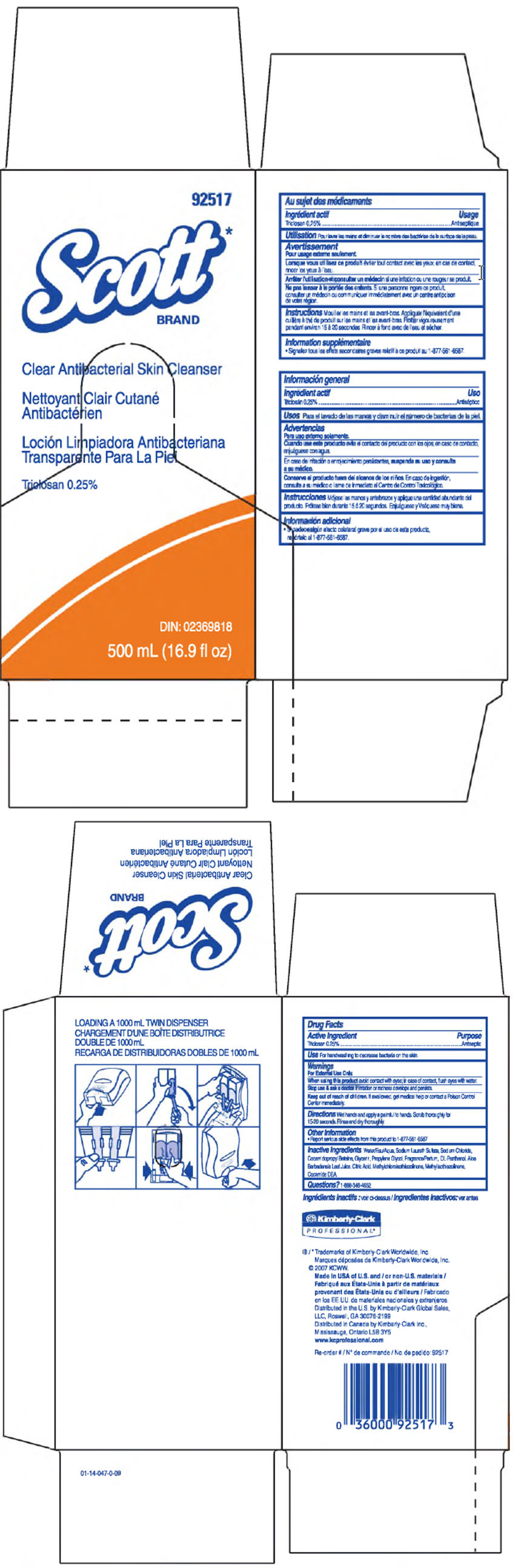
SCOTT CLEAR ANTIBACTERIAL SKIN CLEANSER- triclosan solution
Kimberly-Clark Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Triclosan 0.25%
Use
For handwashing to decrease bacteria on the skin.
Warnings
For External Use Only.
When using this product avoid contact with eyes; in case of contact, flush eyes with water.
Stop use & ask a doctor if irritation or redness develops and persists.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Wet hands and apply a palmful to hands. Scrub thoroughly for 15-20 seconds. Rinse and dry thoroughly.
Other Information
- Report serious side effects from this product to 1-877-561-6587
Inactive Ingredients
Water, Sodium Laureth Sulfate, Sodium Chloride, Cocamidopropyl Beteine, Glycerin, Propylene Glycol, Frangrance, DL-Panthenol, Aloe Barbadensis Leaf Juice, Citric Acid, Methylchloroisothiazolinone, Methylisothiazolinone, Cocamide DEA.
Questions?
1-888-346-4652
Distributed in the U.S. by Kimberly-Clark Global Sales, LLC, Roswell, GA 30076-2199
Distributed in Canada by Kimberly-Clark Inc., Mississauga, Ontario L5B 3Y5
PRINCIPAL DISPLAY PANEL - 500 mL Carton
92517
Scott*
BRAND
Clear Antibacterial Skin Cleanser
Triclosan 0.25%
DIN: 02369818
500 mL (16.9 fl oz)