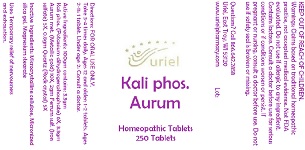
Take 3-4 times daily. Ages 12 and older: 1-2 tablets. Ages 2-11: 1 tablet. Under age 2: Consult a doctor.
Active Ingredients: 100gm contains: 3.3gm Kali phos. (Potassium dihydrogenphosphate) 6X, 3.3gm Aurum met. (Metallic gold) 10X, 2gm Ferrum sulf. (Iron sulfate) 5X, 0.8gm Quartz (Rock crystal) 5X
Warnings: Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated. Do not use if allergic to any ingredient. Contains lactose. Consult a doctor before use for serious conditions or if conditions worsen or persist. If pregnant or nursing, consult a doctor before use. Do not use if safety seal is broken or missing.