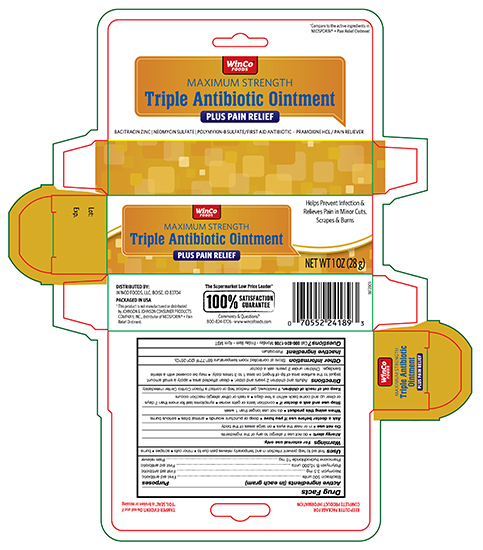
Active ingredients Purpose
Bacitracin zinc 500 units.............................................................................First aid antibiotic
Neomycin sulfate 3.5 mg............................................................................First aid antibiotic
Polymyxin B sulfate 10,000 units.................................................................First aid antibiotic
Pramoxine hydrochloride 10 mg..................................................................External analgesic
Uses
first aid to help prevent infection and for temporary relief of pain or discomfort in minor:
- cuts
- scrapes
- burns
Stop use and ask a doctor if
- you need to use longer than 1 week
- the condition persists or gets worse
- symptoms persist for more than 1 week, or clear up and occur again within a few days
- rash or other allergic reaction develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control right away
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: ask a doctor
Other information
- store between 20° to 25°C (68° to 77°F)
- Lot No. & Exp. Date: see box or see crimp of tube