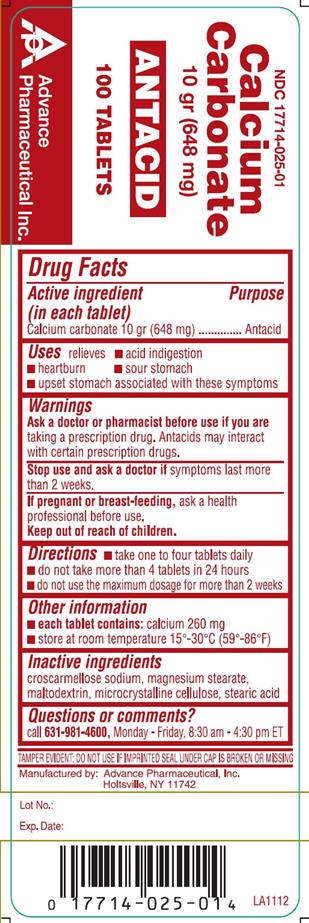
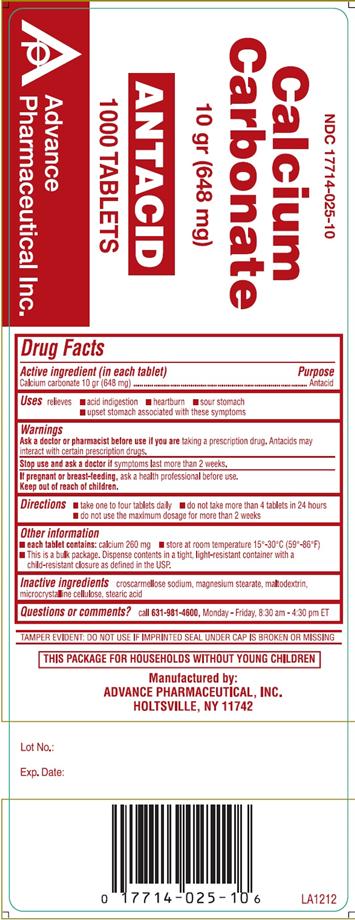
Warnings
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if symptoms last more than 2 weeks.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- take one to four tablets daily.
- do not take more than 4 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks
Other Information
- each tablet contains: calcium 260 mg
- store at room temperature 15-30 °C (59-86 °F)
- For 1000 Count: This is a bulk package. Dispense contents in a tight, light-resistant container with a child-resistant closure as defined in the USP.
Inactive Ingredients
Croscarmellose sodium, magnesium stearate, maltodextrin, microcrystalline cellulose, stearic acid