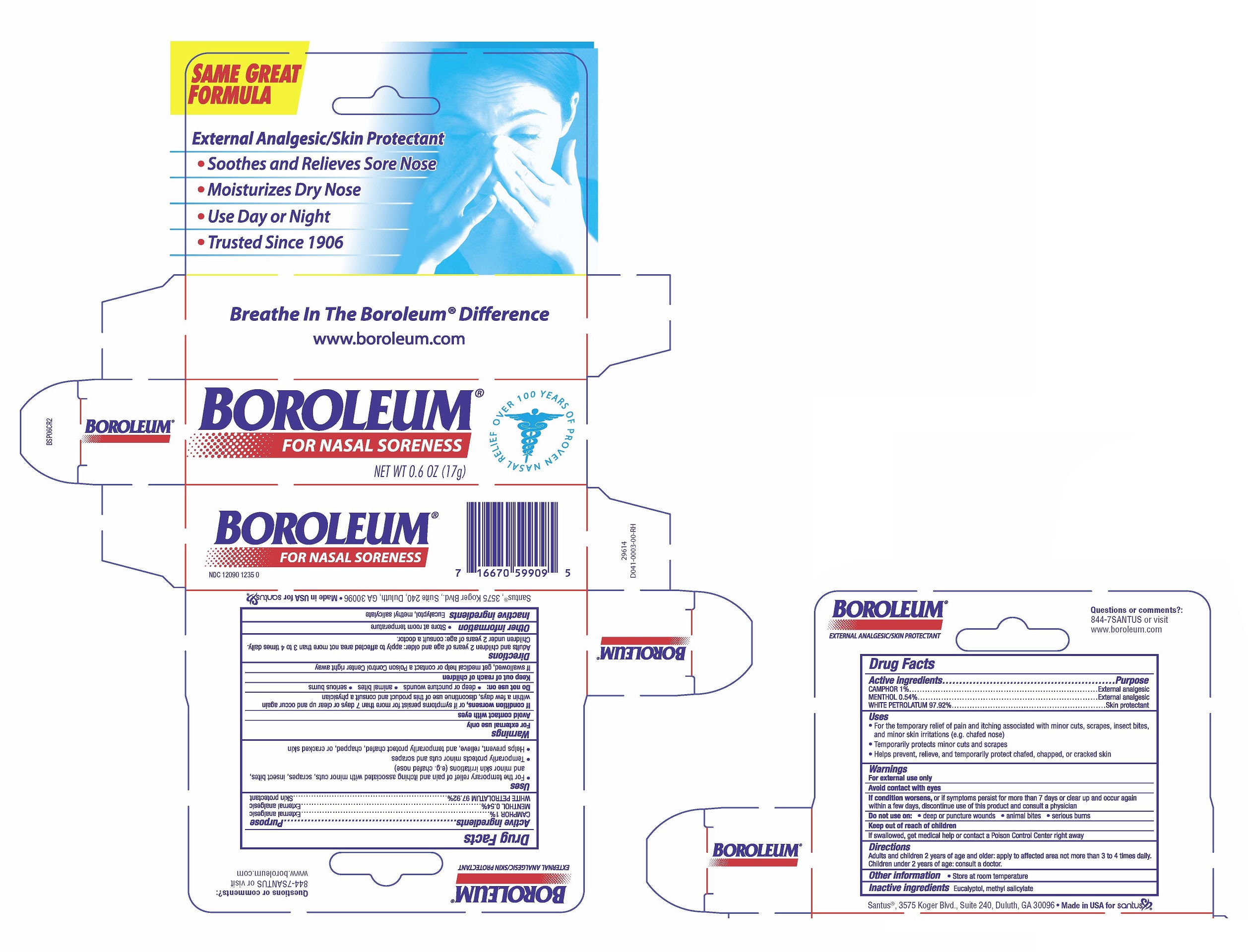
BOROLEUM FOR NASAL SORENESS- camphor, menthol, white petrolatum ointment
Santus LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Boroleum
Uses
- For the temporary relief of pain and itching associated with minor cuts, scrapes, insect bites, and minor skin irritations (e.g. chafed nose)
- Temporarily protects minor cuts and scrapes
- Helps prevent, relieve, and temporarily protect chafed, chapped, or cracked skin
Warnings
For external use only
Avoid contact with eyes
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
| BOROLEUM
FOR NASAL SORENESS
camphor, menthol, white petrolatum ointment |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Santus LLC (079868223) |
Revised: 1/2020
Document Id: 9d4b60a8-b1a9-0b43-e053-2a95a90ae5ce
Set id: 00bd405f-c1c2-402d-bc2e-fa25c8ffaa25
Version: 4
Effective Time: 20200129
Santus LLC