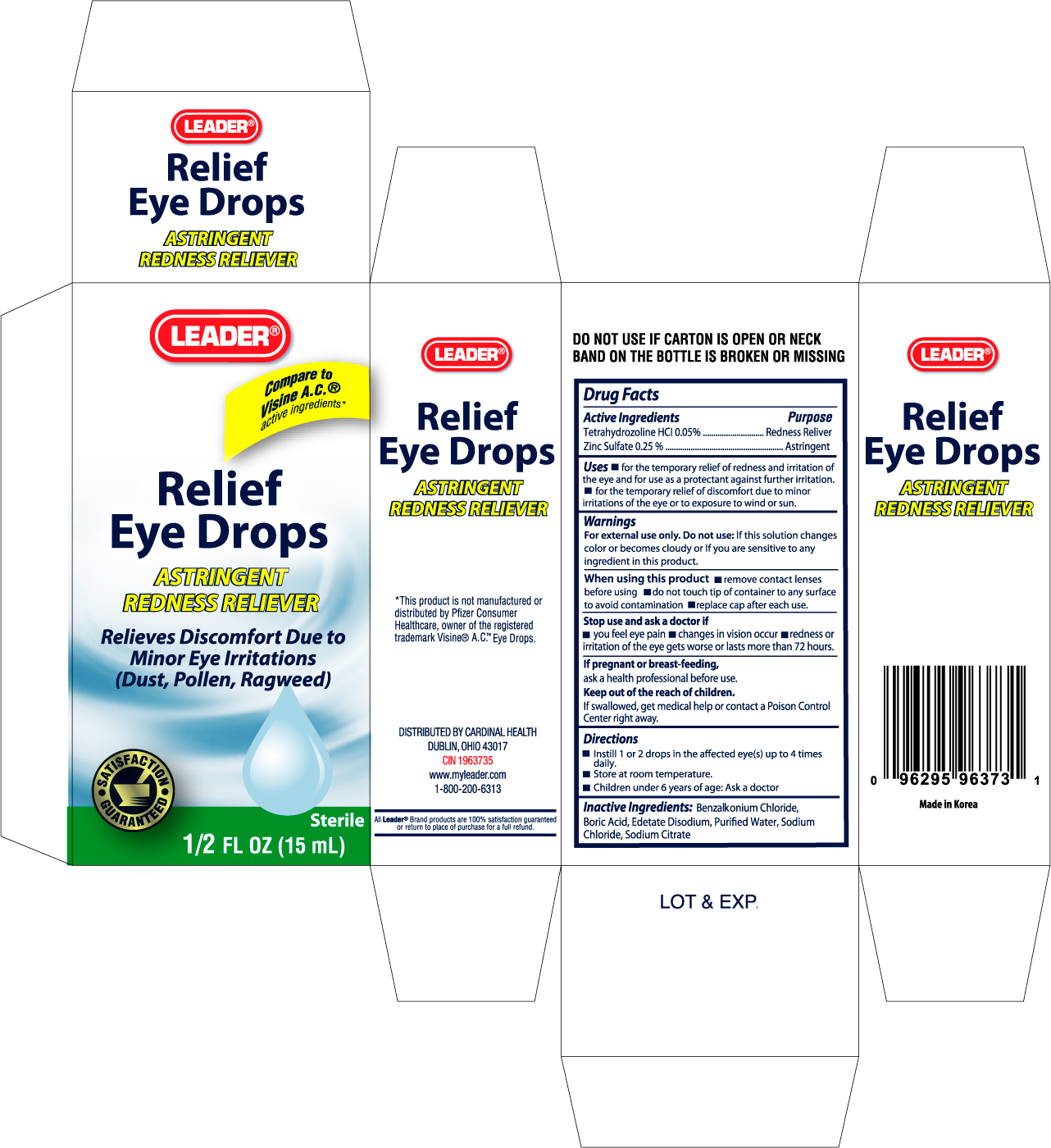
Active ingredients Purpose
Tetrahydrozoline HCL 0.05% ...........................................................Redness Reliever
Zinc Sulfate 0.25% .........................................................................Astringent
Uses
- for the temporary relief of redness and irritation of the eye and for use as a protectant against further irritation.
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun.
Do not use: If this solution changes color or becomes cloudy or if you are sensitive to any ingredient in this product.
When using this product
- remove contact lenses before using
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of eye gets worse or lasts more than 72 hours
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- instill 1 or 2 drops in the affected eye(s) up to 4 times daily.
- Store at room temperature.
- Children under 6 years of age: Ask a doctor
 Enter section text here
Enter section text here