A2506-18 SINGLE SHOT EPIDURAL 18G TUOHY- regional anesthesia kit
Smiths Medical ASD, Inc.
----------
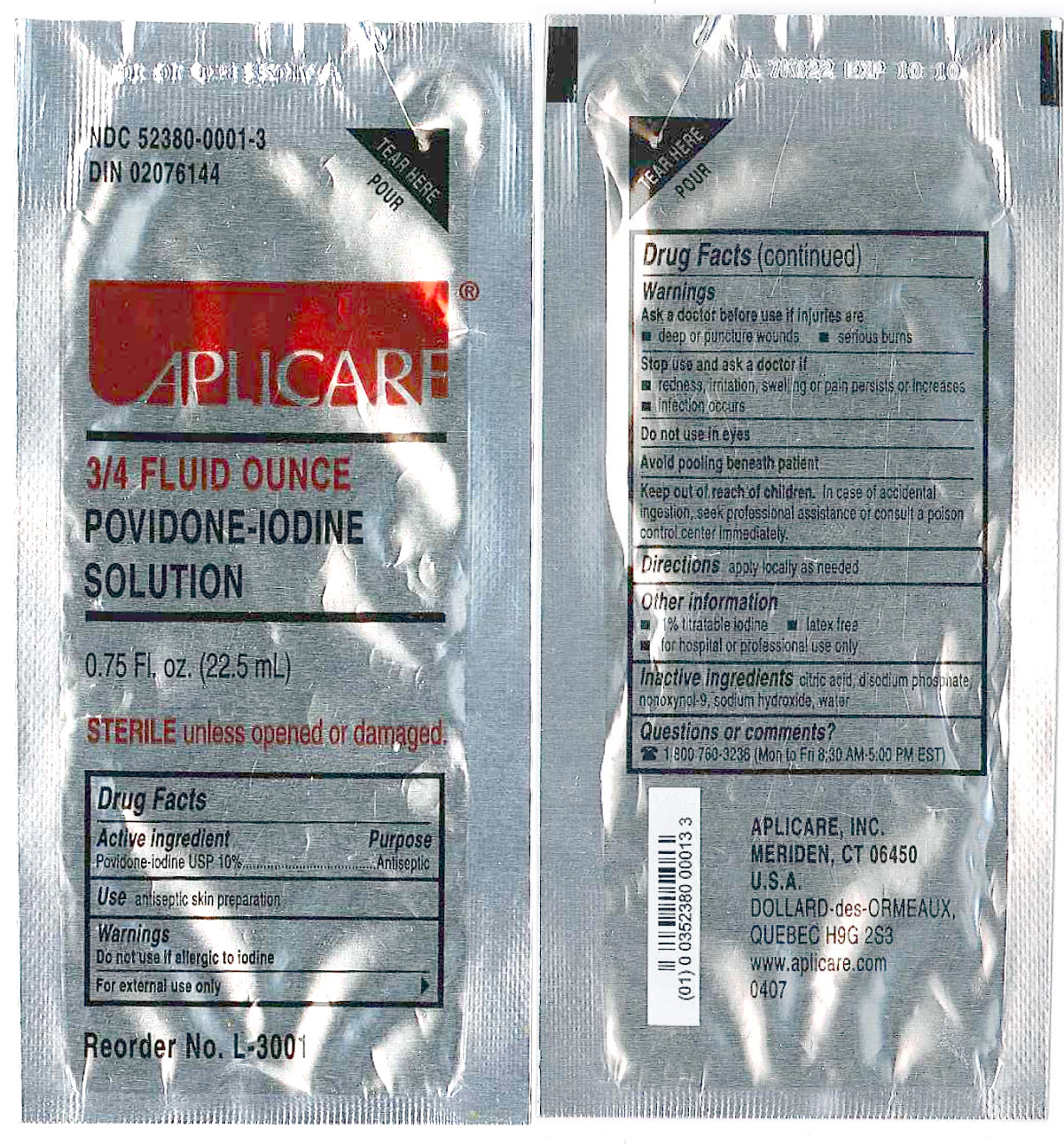
Drug Facts
APLICARE POVIDONE IODINE SOLUTION (povidone-iodine) solution
[Aplicare, Inc.]
3/4 FLUID OUNCE
POVIDONE-IODINE
SOLUTION
0.75 Fl. oz. (22.5)
Active Ingredient
Povidone-iodine
Purpose
Antiseptic
Use
Antispetic skin preparation
Warnings
Do not use
-if allergic to iodine.
-in the eyes
For external use only.
Ask a doctor before use if injuries are
-deep or puncture wounds
-serious burns
Stop use and ask a doctor if
-redness, irritation, swelling or pain persists or increases
-infection occurs
Keep out of reach of children
In case of accidental ingestion, seek professional help or consult a poison control center immediately.
Directions
Apply locally as needed.
Inactive Ingredients
citric acid, disodium phosphate, nonoxynol-9, sodium hydroxide, water
Questions or comments?
1 800 760-3236 (Mon to Fri 8:30 AM-5:00 PM EST)
| A2506-18 SINGLE SHOT EPIDURAL 18G TUOHY
regional anesthesia kit kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Smiths Medical ASD, Inc. (137835299) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smiths Medical ASD, Inc. | 137835299 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare, Inc. | 107255002 | manufacture | |
