Label: EYE STREAM- purified water solution
- NDC Code(s): 0065-0530-01, 0065-0530-04
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
Inactive Ingredients
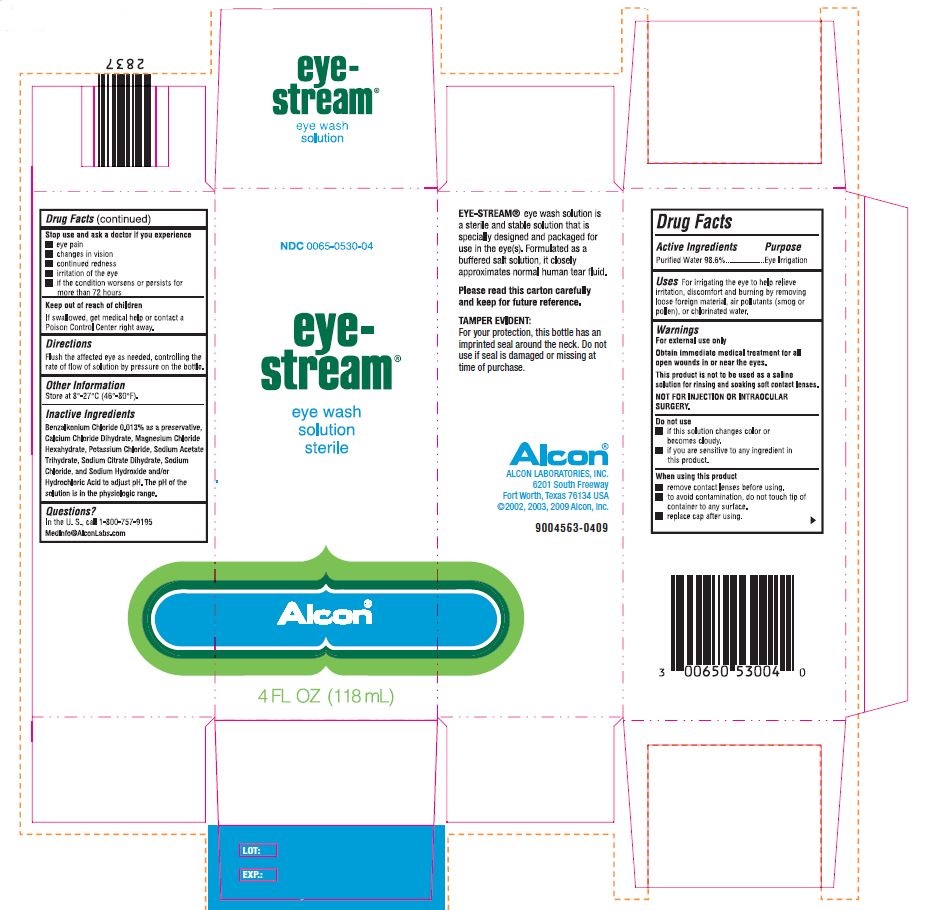
Benzalkonium Chloride 0.013% as preservative, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate, Potassium Chloride, Sodium Acetate Trihydrate, Sodium Chloride, Sodium Citrate Dihydrate, Sodium Hydroxide and/or Hydrochloric Acid to adjust pH. The pH range of solution is in the physiologic range.
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if you experience
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Questions?
- ACTIVE INGREDIENT
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if you experience
- Keep out of reach of children
- Directions
- Other Information
-
Inactive Ingredients
Benzalkonium Chloride 0.013% as a preservative, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate, Potassium Chloride, Sodium Acetate Trihydrate, Sodium Citrate Dihydrate, Sodium Chloride and Sodium Hydroxide and/or Hydrochloric Acid to adjust pH. The pH of the solution is in the physiological range.
- Questions?
-
PRINCIPAL DISPLAY PANEL
NDC 0065-0530-01
eye
stream®
eye wash
solution
sterile
Alcon
1 FL OZ (30 mL)
EYE-STREAM® eye wash
solution is a sterile and stable
solution that is specially
designed and packaged for use
in the eye(s). Formulated as a
buffered salt solution, it closely
approximates normal human
tear fluid.
Please read this carton
carefully and keep for future
reference.
TAMPER EVIDENT: For your
protection, this bottle has an
imprinted seal around the neck.
Do not use if seal is damaged
or missing at time of purchase.
Alcon
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
©2000, 2003, 2010 Alcon, Inc.
300047379-0321
LOT: EXP:

-
PRINCIPAL DISPLAY PANEL
NDC 0065-0530-04
eye-
stream®
eye wash
solution
sterile
Alcon
4 FL OZ (118 mL)
EYE-STREAM® eye wash solution is
a sterile and stable solution that is
specially designed and packaged for
use in the eye(s). Formulated as a
buffered salt solution, it closely
approximates normal human tear fluid.
Please read this carton carefully
and keep for future reference.
TAMPER EVIDENT:
For your protection, this bottle has an
imprinted seal around the neck. Do not
use if seal is damaged or missing at
time of purchase.
Alcon©
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134 USA
© 2000, 2003, 2009 Alcon, Inc.
9004563-0409
LOT:
EXP: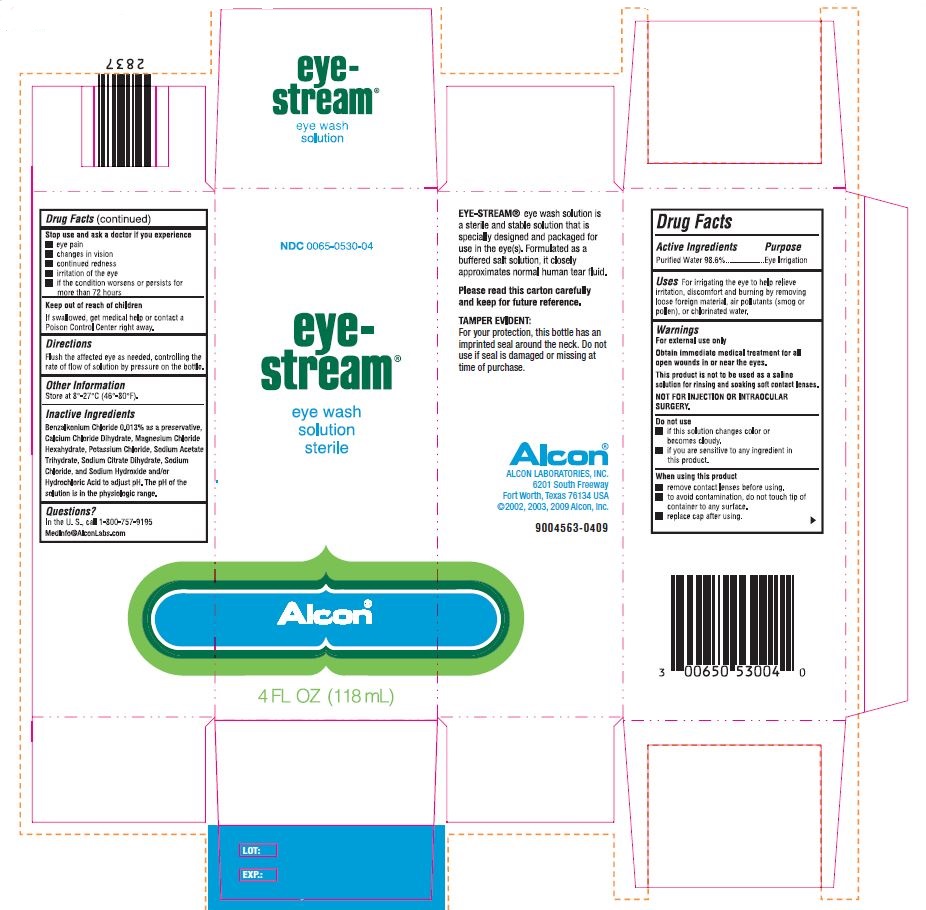
-
INGREDIENTS AND APPEARANCE
EYE STREAM
purified water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-0530 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) Water 986 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Calcium Chloride (UNII: M4I0D6VV5M) Magnesium Chloride (UNII: 02F3473H9O) Potassium Chloride (UNII: 660YQ98I10) Sodium Acetate (UNII: 4550K0SC9B) Sodium Chloride (UNII: 451W47IQ8X) Trisodium Citrate Dihydrate (UNII: B22547B95K) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0530-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/30/1990 2 NDC:0065-0530-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/30/1990 05/31/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 09/30/1990 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0530)