Label: ULTRACARE ORAL ANESTHETIC BUBBLE GUM- benzocaine gel
- NDC Code(s): 51206-201-01, 51206-201-02
- Packager: Ultradent Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Methemoglobinemia warning
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy.
For external use only
Allergy alert
Do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics, PABA compounds, or sunscreen. If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning
if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, or if irritation, pain, or redness persist or worsens, see your dentist or doctor promptly.
-
Directions
Adults and children 2 years of age and older Apply a thin film to affected area. Allow to remain in place at least 1 minute and then spit out. Use up to 4 times daily or as directed by your dentist or doctor. Children between 2 and 12 years of age Should be supervised in the use of this product. Children under 2 years of age Do not use. - Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Ultracare®
ORAL ANESTHETIC GELDescription:
Ultracare is a 20% w/v (17.9%) benzocaine oral anesthetic gel preparation in a water-soluble glycol base. Ultracare is designed for rapid onset (10-30 seconds). Anesthesia usually lasts 8-10 minutes. Ultracare is NOT for injection.
Uses:
For the temporary relief of occasional minor irritation and pain, associated with:
- Minor dental procedures
- Sore mouth and throat
- Minor injury of the gums
- Canker sores
- Minor irritation of the mouth or gums caused by dentures or orthodontic appliances
Warnings:
Methemoglobinemia warning:
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy.
For external use only
Allergy alert:
Do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics, PABA compounds, or sunscreen. If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning:
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, or if irritation, pain, or redness persist or worsens, see your dentist or doctor promptly.
Procedure:
- 1.
- Preloaded 1.2ml syringes:
- a.
- Remove luer lock cap from syringe.
- b.
- Attach a Blue Micro® 20-gauge Tip or 5mm Micro Capillary Tip securely onto syringe.
- c.
- Slowly express gel to the target tissue.
- d.
- For anesthetizing periodontal pockets:
- i.
- Slowly apply a small amount to one quadrant at a time to the gingival margin or directly into the periodontal pockets and proceed with sub-gingival scaling.
Fig. 1 Hold plunger in palm of hand for optimum control.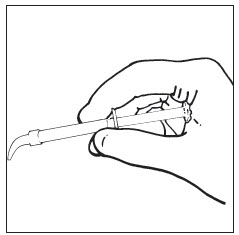
Fig. 2 Deliver Ultracare from the large, no-waste IndiSpense syringe to cotton applicator, cotton roll, etc., for placement.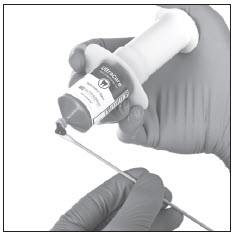
- 2.
- IndiSpense® delivery:
- a.
- Remove cap from IndiSpense syringe.
- b.
- Express desired amount of anesthetic to cotton applicator (Fig. 2) or into a dappen dish. Use a clean applicator with each area application. Re-cap the IndiSpense syringe after each use.
- c.
- Using a dappen dish allows for re-application with the same applicator without the risk of cross-contamination. Re-cap the IndiSpense syringe after each use.
- 3.
- IndiSpense delivery to empty 1.2ml syringe:
- a.
- Attach a 1.2ml syringe to the end of IndiSpense by turning the luer lock of the 1.2ml syringe snugly onto male thread of Indispense syringe.
- b.
- With palm, press plunger of IndiSpense syringe gently while guiding the 1.2ml plunger outward to desired fill.
- c.
- RE-cap the IndiSpense syringe after each use.
- d.
- See instruction for 1.2ml syringe use.
Directions:
Adults and children 2 years of age and older:
- Apply a thin film to affected area. Allow to remain in place at least 1 minute and then spit out. Use up to 4 times daily or as directed by your dentist or doctor.
- Rinse and expectorate to clear the oral cavity of residual product.
- Apply a thin film to injection site(s) allowing the gelled cotton applicator to remain in place at least 1 minute.
- Rinse and suction the oral cavity and continue with administering local anesthetic or with dental procedure.
For anesthetizing periodontal pockets:
- See instruction for 1.2ml use.
Children between 2 and 12 years of age:
- Should be supervised in the use of this product
Children under 2 years of age:
- Do not use
Warnings and Precautions:
- 1.
- For Professional use only.
- 2.
- For external use only.
- 3.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- 4.
- Ultracare is NOT for injection.
- 5.
- Pregnancy and Lactation: Safety is not known in pregnancy/nursing women.
- 6.
- Avoid contact with eyes.
- 7.
- If applying intraorally from a syringe, verify flow on a gauze pad or mixing pad prior to applying. If resistance is met, replace tip and re-check.
- 8.
- If an excessive amount of gel is swallowed, get medical help or contact a Poison Control Center immediately.
- 9.
- Do not exceed maximum recommended dosage.
- 10.
- Phenylketonurics: Contains Phenylalanine—3mg per gram
- 11.
- See state or country guidelines for proper disposal of empty jar, syringes, applicator and tips.
- 12.
- To avoid cross-contamination of the prefilled syringes, use a disposable syringe cover, re-cap, and wipe syringe with an intermediate disinfectant between uses. If these measures are not taken, the syringes should be considered single-use. Tips are disposable. To avoid cross-contamination, do not re-use tips.
For immediate reorder and/or complete descriptions of Ultradent's product line, refer to Ultradent's catalog or call Toll Free:
1-800-552-5512.
Outside U.S. call (801) 572-4200.
All Ultradent syringes have an expiration date stamped on the side of the syringe consisting of one letter and three numbers. The letter is a lot number used for manufacturing purposes and the three numbers are the expiration date. The first two numbers are the month, and the third number is the last number of the year. 
© 2018 Ultradent Products, Inc. All Rights Reserved. Made in U.S.A.
Manufactured by Ultradent Products, Inc. 505 West Ultradent Drive, (10200 South) South Jordan, UT 84095 10057.18 052318 - PRINCIPAL DISPLAY PANEL - 30 mL Syringe Label
-
INGREDIENTS AND APPEARANCE
ULTRACARE ORAL ANESTHETIC BUBBLE GUM
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51206-201 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength Aspartame (UNII: Z0H242BBR1) Alcohol (UNII: 3K9958V90M) FD&C Red No. 40 (UNII: WZB9127XOA) Glycerin (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Saccharin Sodium (UNII: SB8ZUX40TY) Titanium Dioxide (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51206-201-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1986 06/18/2023 2 NDC:51206-201-02 1 in 1 BOX 06/01/1986 08/31/2024 2 30 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 06/01/1986 08/31/2024 Labeler - Ultradent Products, Inc. (013369913) Establishment Name Address ID/FEI Business Operations Ultradent Products, Inc. 013369913 MANUFACTURE(51206-201) , ANALYSIS(51206-201) , LABEL(51206-201) , PACK(51206-201)


