DR BOB ARNOTS PAIN PEN- camphor (natural) liquid
Larasan Pharmaceutical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
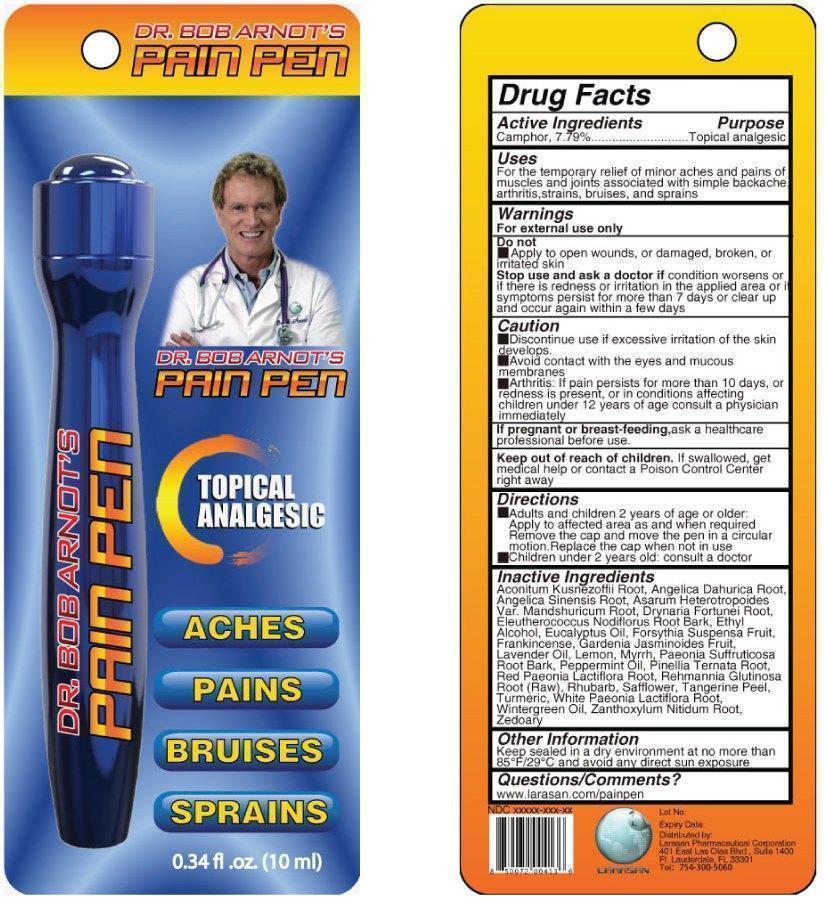
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises, and sprains
Warnings
For external use only.
Stop Use and Ask a Doctor If
Condition worsens, or if there is redness or irritation in the applied area or if symptoms persist for more than 7 days or clear up and occur again within a few days
Directions
- Adults and children 2 years of age and older: Apply to affected area as needed and when required. Remove the cap and move the pen in a circular motion. Replace cap when not in use.
- Children under 2 years of age: consult a doctor.
Inactive Ingredients
Aconitum Kusnezoffii Root, Angelica Dahurica Root, Angelica Sinesis Root, Asarum Heterotropoides var. Mandshuricum Root, Drynaria Fortunei Root, Eleutherococcus Nodiflorus Root Bark, Ethyl Alcohol, Eucalyptus Oil, Frosythia Suspensa Fruit, Frankincense, Gardenia Jasminoides Fruit, Lavender Oil, Lemon, Myrrh, Paeonia Suffruticosa Root Bark, Peppermint Oil, Pinellia Ternata Root, Red Paeonia Lactiflora Root, Rehmannia Glutinosa Root (Raw), Rhubarb, Safflower, Tangerine Peel, Turmeric, White Paeonia Lactiflora Root, Wintergreen Oil, Zanthoxylum Nitidum Root, Zeodary
Other Information
Keep sealed in a dry environment at no more than 85°F / 29°C and avoid any direct sun exposure
| DR BOB ARNOTS PAIN PEN
camphor (natural) liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Larasan Pharmaceutical Corporation (078350375) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dong-e E-jiao E-hua Medical Equipment Co Ltd | 527813247 | manufacture(54641-002) | |