ANTIPYRINE AND BENZOCAINE - antipyrine and benzocaine solution
REMEDYREPACK INC.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Antipyrine and Benzocaine Otic Solution USP Ear SolutionTopical Decongestant and AnalgesicRx Only
DESCRIPTION
Antipyrine and Benzocaine Otic Solution is an otic solution containing Antipyrine, Benzocaine, Oxyquinoline Sulfate, and Anhydrous Glycerin for use in the ear. The solution congeals at 0°C (32°F), but returns to normal consistency, unchanged, at room temperature.
Antipyrine is an analgesic with local anesthetic action, it is chemically 2,3-dimethyl-1-phenyl-3-pyrazolin-5-one. The active ingredient is represented by the structural formula:
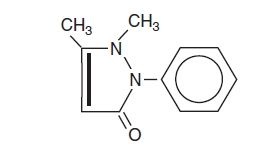
C11H12N2O
Mol. Wt. 188.22
Antipyrine occurs as colorless crystals or white powder, has a slightly bitter taste and is soluble in water and alcohol.
Benzocaine is a local anesthetic. It is chemically ethyl p-aminobenzoate or Benzoic acid, 4-amino-, ethyl ester. The active ingredient is represented by the structural formula:
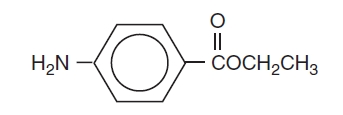
C9H11NO2
Mol. Wt. 165.19
It occurs as white crystals or white crystalline powder and is slightly soluble in water and soluble in organic solvents.
Each mL Contains: ACTIVES: Antipyrine 54 mg (5.4%), Benzocaine 14 mg (1.4%); INACTIVES: Glycerin, Oxyquinoline Sulfate.
CLINICAL PHARMACOLOGY
Antipyrine and Benzocaine Otic Solution combines the hygroscopic property of anhydrous glycerin with the analgesic action of antipyrine and benzocaine to relieve pressure, reduce inflammation and congestion, and to alleviate pain and discomfort in acute otitis media.
Antipyrine and Benzocaine Otic Solution does not blanch the tympanic membrane or mask the landmarks and, therefore, does not distort the otoscopic picture.
INDICATIONS & USAGE
Acute Otitis media of various etiologies
-prompt relief of pain and reduction of inflammation in the congestive and serous stages.
-adjuvant therapy during systemic antibiotic administration for resolution of the infection.
Because of the close anatomical relationship of the eustachian tube to the nasal cavity, otitis media is a frequent problem, especially in children in whom the tube is shorter, wider, and more horizontal than in adults.
Removal of Cerumen
-facilitates the removal of excessive or impacted cerumen.
CONTRAINDICATIONS
The product is contraindicated in any person with hypersensitivity to any of the components or substances related to them. This product is contraindicated in the presence of spontaneous perforation of the tympanic membrane or discharge.
WARNINGS
FOR USE IN EARS ONLY- NOT FOR USE IN EYES
Discontinue promptly if sensitization or irritation occurs.
PRECAUTIONS
Avoid contaminating the dropper with material from the ear, fingers or other source.
No long term studies have been conducted.
Category C. Animal reproduction studies have not been conducted with Antipyrine and Benzocaine Otic Solution. It is also not known whether Antipyrine and Benzocaine Otic Solution can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Antipyrine and Benzocaine Otic Solution should be given to a pregnant woman only if clearly needed.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Antipyrine and Benzocaine Otic Solution is administered to a nursing woman.
DOSAGE & ADMINISTRATION
Acute otitis media: Instill Antipyrine and Benzocaine Otic Solution, permitting the solution to run along the wall of the ear canal until it is filled. Avoid touching the ear with dropper. Then moisten a cotton pledget with Antipyrine and Benzocaine Otic Solution and insert into meatus. Repeat every one to two hours until pain and congestion are relieved.
Removal of Cerumen:
Before: Instill Antipyrine and Benzocaine Otic Solution three times daily for two or three days to help detach cerumen from wall of ear canal and facilitate removal.
After: Antipyrine and Benzocaine Otic Solution is useful for drying out the ear canal or relieving discomfort.
Before and after removal of cerumen, a cotton pledget moistened with Antipyrine and Benzocaine Otic Solution should be inserted into the meatus following instillation.
Use of Dropper:
Remove cap from bottle and discard. Remove dropper from package. Place dropper in bottle and use as directed. After use, do not rinse dropper and place dropper in bottle and close tightly.
NOTE: Do not use if solution is brown or contains a precipitate.
DISCARD THIS PRODUCT SIX MONTHS AFTER DROPPER IS FIRST PLACED IN THE DRUG SOLUTION.
HOW SUPPLIED
Antipyrine and Benzocaine Otic Solution USP is supplied in an amber glass bottle with a dropper in the following size:
10 mL - Prod. No. 12409

Storage: Store between 15°-25°C (59°- 77°F). PROTECT FROM LIGHT AND HEAT.
KEEP OUT OF REACH OF CHILDREN.
Revised February 2008
Bausch & Lomb Incorporated
Tampa, FL 33637
©Bausch & Lomb Incorporated
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
DRUG: Antipyrine and Benzocaine
GENERIC: Antipyrine and Benzocaine
DOSAGE: SOLUTION
ADMINSTRATION: AURICULAR (OTIC)
NDC: 61786-328-08
ACTIVE INGREDIENT(S):
- ANTIPYRINE 54mg in 1mL
- BENZOCAINE 14mg in 1mL
INACTIVE INGREDIENT(S):
- GLYCERIN
- OXYQUINOLINE SULFATE
PACKAGING: 10 mL in 1 BOTTLE, DROPPER

| ANTIPYRINE AND BENZOCAINE
antipyrine and benzocaine solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |