CALGEST- calgest tablet
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
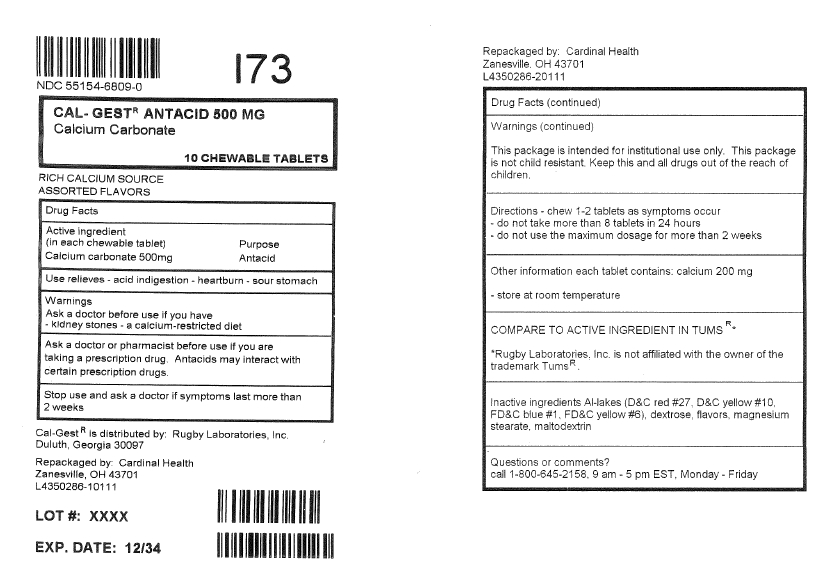
CalGest Antacid Tablets
Warnings
Ask a doctor before use if you have
- •
- Kidney stones
- •
- a calcium-restricted diet
- Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
- Stop use and ask a doctor if symptoms last more than 2 weeks.
- Keep out of reach of children.
Directions
- •
- chew 1-2 tablets as symptoms occur
- •
- do not take more than 8 tablets in 24 hours
- •
- do not use the maximum dosage for more than 2 weeks
Inactive Ingredients
Al-lakes (D&C red #27, D&C yellow #10, FD&C blue #1, FD&C yellow #6), dextrose, flavors, magnesium stearate, maltodextrin
Questions or Comments
Call 1-800-645-2158, Monday – Friday, 9 am – 5 pm ET
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL UNDER CAP IS BROKEN OR MISSING
NDC 0536-3742-97
Rugby
Cal.Gest Antacid
150 Chewable Tablets
| CALGEST
calgest tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Cardinal Health (188557102) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health | 188557102 | REPACK(55154-6809) | |
Revised: 4/2018
Document Id: 47aed505-b706-44e8-8bbe-60b8be0343b9
Set id: f9d457d2-6714-4141-be0b-376c85ce10ee
Version: 2
Effective Time: 20180416
Cardinal Health