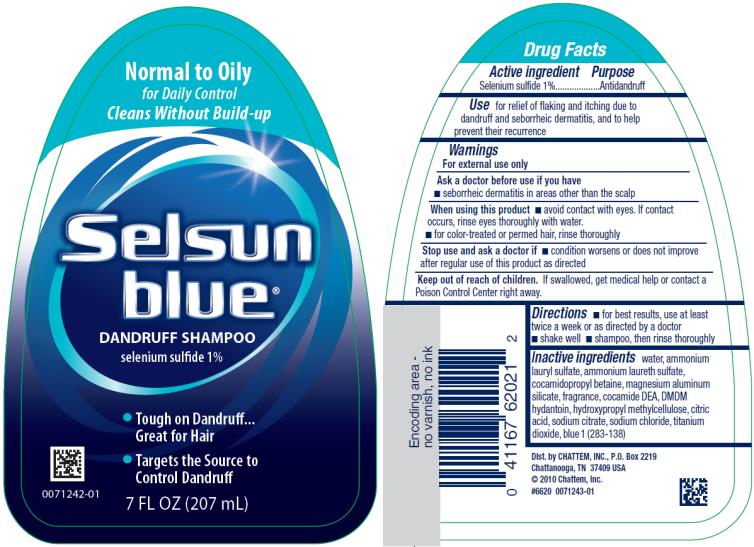
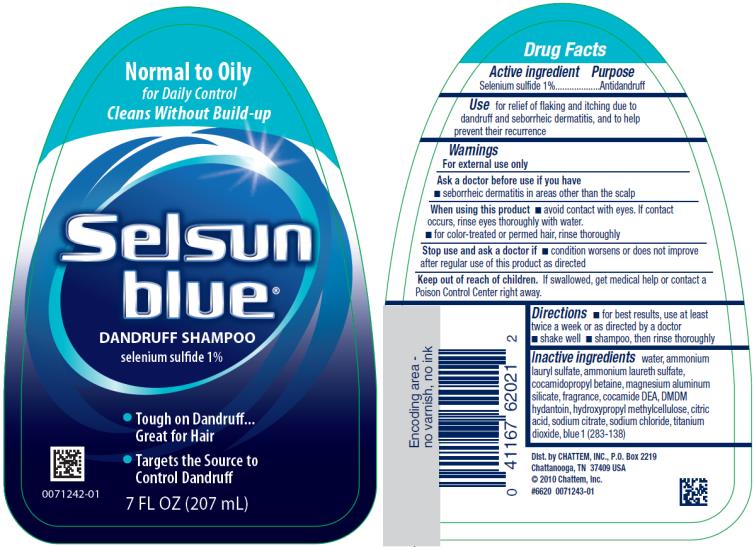
Label: SELSUN BLUE NORMAL TO OILY- selenium sulfide shampoo
SELSUN BLUE MOISTURIZING- selenium sulfide shampoo
SELSUN BLUE 2-IN-1- selenium sulfide shampoo
SELSUN BLUE MEDICATED- selenium sulfide shampoo
-
NDC Code(s):
41167-1651-4,
41167-1651-5,
41167-6032-0,
41167-6032-2, view more41167-6061-1, 41167-6061-2, 41167-6061-3, 41167-6061-6, 41167-6202-1, 41167-6202-3
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
-
Inactive ingredients
water, ammonium lauryl sulfate, distearyl phthalic acid amide, ammonium laureth sulfate, sodium chloride, cocamide DEA, dimethicone, aloe barbadensis leaf juice, hydroxypropyl methylcellulose, sodium isostearoyl lactylate, DMDM hydantoin, fragrance, citric acid, sodium citrate, titanium dioxide, blue 1 (283-134)
Drug Facts
Selsun Blue 2-in-1 - Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SELSUN BLUE NORMAL TO OILY
selenium sulfide shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-6202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM SULFIDE - UNII:Z69D9E381Q) SELENIUM SULFIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) AMMONIUM LAURETH-3 SULFATE (UNII: 896SJ235FN) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) COCO DIETHANOLAMIDE (UNII: 92005F972D) DMDM HYDANTOIN (UNII: BYR0546TOW) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-6202-1 207 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 02/22/2021 2 NDC:41167-6202-3 325 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 02/22/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 04/01/2002 02/22/2021 SELSUN BLUE MOISTURIZING
selenium sulfide shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-6032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM SULFIDE - UNII:Z69D9E381Q) SELENIUM SULFIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) DISTEARYL PHTHALAMIC ACID (UNII: 5552GSZ9LI) AMMONIUM LAURETH-3 SULFATE (UNII: 896SJ235FN) SODIUM CHLORIDE (UNII: 451W47IQ8X) COCO DIETHANOLAMIDE (UNII: 92005F972D) DIMETHICONE (UNII: 92RU3N3Y1O) ALOE VERA LEAF (UNII: ZY81Z83H0X) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SODIUM ISOSTEAROYL LACTYLATE (UNII: 8730J0D3EV) DMDM HYDANTOIN (UNII: BYR0546TOW) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-6032-0 207 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 2 NDC:41167-6032-2 325 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 04/01/2002 SELSUN BLUE 2-IN-1
selenium sulfide shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-1651 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM SULFIDE - UNII:Z69D9E381Q) SELENIUM SULFIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) DISTEARYL PHTHALAMIC ACID (UNII: 5552GSZ9LI) AMMONIUM LAURETH-3 SULFATE (UNII: 896SJ235FN) SODIUM CHLORIDE (UNII: 451W47IQ8X) COCO DIETHANOLAMIDE (UNII: 92005F972D) DIMETHICONE (UNII: 92RU3N3Y1O) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) DMDM HYDANTOIN (UNII: BYR0546TOW) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-1651-5 207 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 01/31/2017 2 NDC:41167-1651-4 325 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 04/01/2002 SELSUN BLUE MEDICATED
selenium sulfide shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-6061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM SULFIDE - UNII:Z69D9E381Q) SELENIUM SULFIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) TRIETHANOLAMINE LAURYL SULFATE (UNII: E8458C1KAA) AMMONIUM LAURETH-3 SULFATE (UNII: 896SJ235FN) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) MENTHOL (UNII: L7T10EIP3A) COCO DIETHANOLAMIDE (UNII: 92005F972D) DMDM HYDANTOIN (UNII: BYR0546TOW) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-6061-6 207 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 2 NDC:41167-6061-2 325 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 3 NDC:41167-6061-3 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2002 06/05/2018 4 NDC:41167-6061-1 325 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 04/01/2002 Labeler - Chattem, Inc. (003336013) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 830410721 label(41167-6202, 41167-6032, 41167-1651, 41167-6061) , manufacture(41167-6202, 41167-6032, 41167-1651, 41167-6061) , pack(41167-6202, 41167-6032, 41167-1651, 41167-6061)