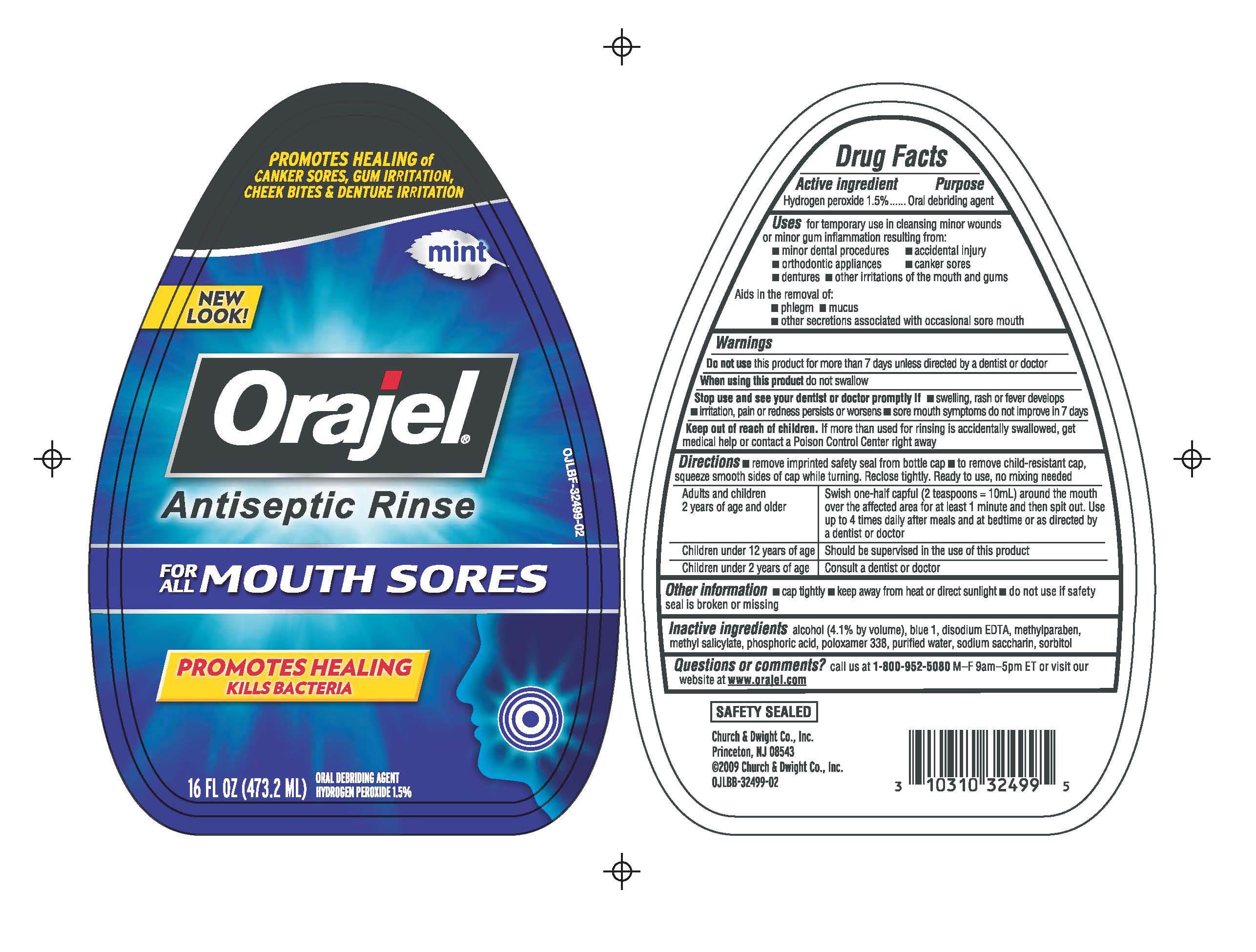
ORAJEL ANTISEPTIC RINSE FOR MOUTH SORES- hydrogen peroxide liquid
Church & Dwight Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Orajel Antiseptic Mouth Sore Rinse
Uses
for temporary use in cleansing minor wounds or minor gum inflammation resulting from:
- minor dental procedures
- orthodontic appliances
- dentures
- accidental injury
- canker sores
- other irritations of the mouth and gums
Aids in the removal of:
- phlegm
- mucus
- other secretions associated with occasional sore mouth
Warnings
Do not use this product for more than 7 days unless directed by a dentist or doctor
When using this product do not swallow
Stop use
Stop use and see your dentist or doctor promptly if
- swelling, rash or fever develops
- irritation, pain or redness persists or worsens
- sore mouth symptoms do not improve in 7 days
Keep out of reach of children
If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away
Directions
- remove imprinted safety seal from bottle cap
- to remove child-resistant cap, squeeze smooth sides of cap while turning. Reclose tightly. Ready to use, no mixing needed.
Adults and children 2 years of age and older
- Swish one-half capful (2 teaspoons = 10 mL) around the mouth over the affected area for at least 1 minute and then spit out. Use up to 4 times daily after meals and at bedtime or as directed by a dentist or doctor
Children under 12 years of age
- Should be supervised in the use of this product
Children under 2 years of age
- Consult a dentist or doctor
Other information
- cap tightly
- keep away from heat or direct sunlight
- do not use if safety seal is broken or missing
Inactive ingredients
alcohol (4.1% by volume), blue 1, disodium EDTA, methylparaben, methyl salicylate, phosphoric acid, poloxamer 338, purified water, sodium saccharin, sorbitol
| ORAJEL ANTISEPTIC RINSE FOR MOUTH SORES
hydrogen peroxide liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Church & Dwight Co., Inc. (001211952) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Accupac, Inc. | 071609663 | manufacture(10237-720) | |