Label: FLUORISHIELD- sodium fluoride gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 10733-130-04 - Packager: Medical Products Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 11, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Your dentist has selected for you FluoriShield, a 1.1% Sodium Fluoride product that was specially developed to prevent tooth decay which can occur from xerostomia (dry mouth). Dry mouth can result from radiation therapy, systemic diseases, medications which affect the salivary glands and mouth breathing. Clinical trials have shown that application of topical fluoride gel in the presence of good oral hygiene can prevent or significantly reduce tooth decay which is related to dry mouth. FluoriShield topical fluoride gel can be applied with a custom carrier or tray, directly brushed on, or used with an over denture. Your dentist will prescribe the method that is best suited for your individual needs. The gel is easily dispensed when the bottle is inverted for storing.
STORE INVERTED FOR EASY REMOVAL OF CONTENTS.
- Description:
- Warnings:
- Overdose:
- STORAGE AND HANDLING
- CAUTION:
-
INSTRUCTIONS
Custom Carriers or Trays
Brush and floss your teeth to remove plaque. Adults and children 6 years of age or older: Add one drop of FluoriShield into each tooth area in the custom carrier and spread evenly with the applicator tip of the fluoride bottle. Place carriers over the teeth and let it remain in place for one (1) minute or longer (as directed by your doctor). Expectorate the excess gel. Clean carriers with cold water. Spit out the excess gel in the mouth and do not rinse, eat, or drink for thirty (30) minutes.
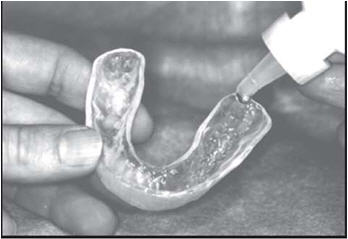
Brush On
Brush and floss your teeth to remove plaque. Adults and children 6 years of age or older: Apply a thin ribbon of gel to the toothbrush and spread evenly with the applicator tip of the fluoride bottle. Brush all tooth surfaces and allow fluoride to remain in place for one (1) minute or longer (as directed by your doctor). Clean carriers with cold water. Expectorate the excess gel in the mouth and do not rinse, eat, or drink for thirty (30) minutes.
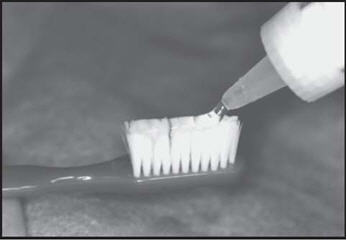
Overdentures
Brush all tooth surfaces. Clean your dentures. Adults and children 6 years of age or older: Apply one (1) or two (2)drops of FluoriShield to each tooth area in your denture and place the dentures in your mouth. Leave in place for one (1) minute or longer (as directed by your doctor).Expectorate the excess gel in the mouth and do not rinse, eat, or drink for thirty (30) minutes.
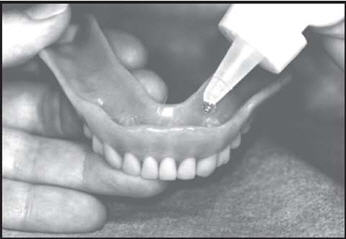
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUORISHIELD
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10733-130 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 11 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) HYDROXYETHYL CELLULOSE (4000 MPA.S FOR 1% AQUEOUS SOLUTION) (UNII: ZYD53NBL45) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10733-130-04 66 in 1 CARTON 1 114 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/29/2009 Labeler - Medical Products Laboratories, Inc. (002290302)


