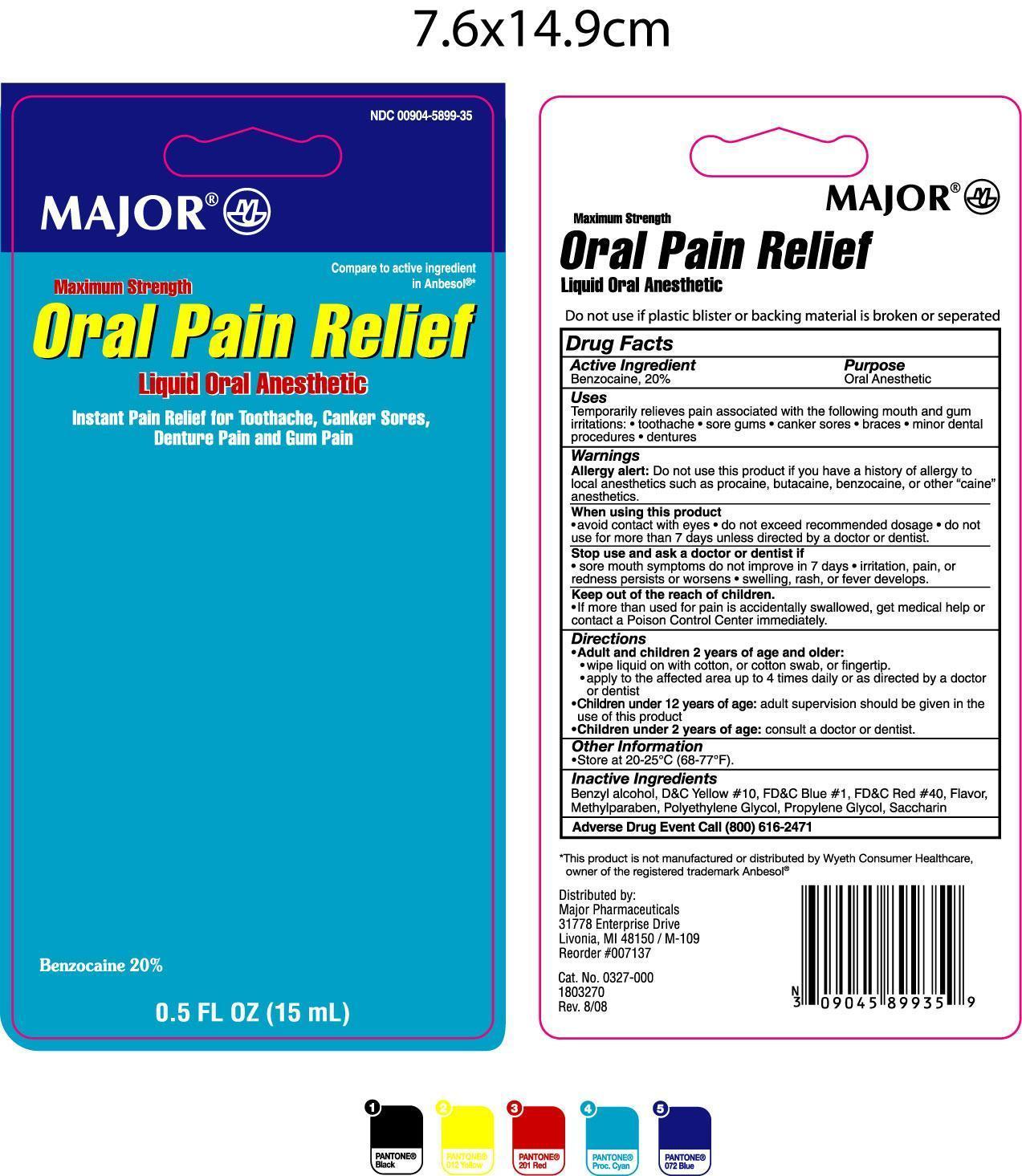
MAJOR ORAL PAIN RELIEF MAXIMUM STRENGTH- oral anesthetic liquid
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
Temporarily relieves pain associated with the following mouth and gum irritations:
- •
- toothache
- •
- sore gums
- •
- canker sores
- •
- braces
- •
- minor dental procedures
- •
- dentures
Warnings
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
When using this product
- •
- avoid contact with eyes
- •
- do not exceed recommended dosage
- •
- do not use for more than 7 days unless directed by a doctor or dentist
Stop use and ask a doctor or dentist if
- •
- sore mouth symptoms do not improve in 7 days
- •
- irritation, pain, or redness persists or worsens
- •
- swelling, rash, or fever develops
Keep out of reach of children
- •
- If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center immediately
Directions
- •
- Adults and children 2 years of age and older:
- •
- wipe liquid on with cotton, or cotton swab, or finger tip.
- •
- apply to the affected area up to 4 times daily of as directed by a doctor or dentist.
- •
- Children under 12 years of age: adult supervision should be given in the use of this product.
- •
- Children under 2 years of age: consult a doctor or dentist.
| MAJOR ORAL PAIN RELIEF
MAXIMUM STRENGTH
oral anesthetic liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
Revised: 1/2020
Document Id: 209a1af2-e230-423f-82ec-50d54a98bfb6
Set id: f659c896-815e-4c58-8fa7-f1356955973e
Version: 5
Effective Time: 20200117
Major Pharmaceuticals