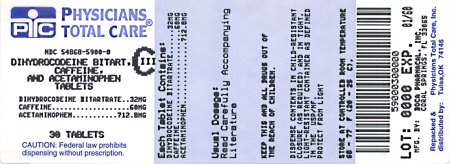
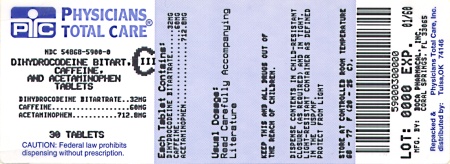
Label: DIHYDROCODEINE BITARTRATE, ACETAMINOPHEN AND CAFFEINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5900-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 64376-611
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 24, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets are supplied in tablet form for oral administration.
Each tablet contains:
Acetaminophenִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִ712.8 mg
Caffeineִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִִ60 mg
Dihydrocodeine* bitartrateִִִִִִִִִִִִִִִִִִִִִִ32 mg
*Warning: May be habit formingAcetaminophen (4'-hydroxyacetanilide), a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic.
It has the following structural formula:
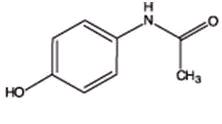
C8H9NO2 M.W.=151.16
Caffeine (1,3,7-trimethylxanthine), a bitter, white crystalline powder, is a central nervous system stimulant. It has the following structural formula:
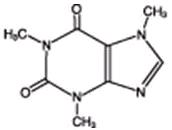
C8H10N4O2 M.W=194.19
Dihydrocodeine Bitartrate (4,5a-epoxy-3-methoxy-17-methylmDihydrocodeine Bitartrate (4,5a-epoxy-3-methoxy-17-methylmDihydrocodeine Bitartrate (4,5a-epoxy-3-methoxy-17-methylmDihydrocodeine Bitartrate (4,5a-epoxy-3-methoxy-17-methylmorphinan-6a-ol (+)-tartrate), an odorless, fine white powder is an opioid analgesic. It has the following structural formula:
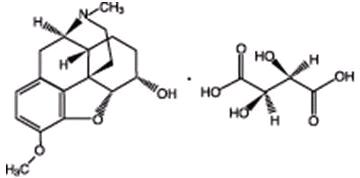
C18H23NO3 C4H6O6 M.W.=451.47
In addition, each tablet also contains the following inactive ingredients: crospovidone, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, stearic acid.
-
CLINICAL PHARMACOLOGY:
Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets contain dihydrocodeine which is a semisynthetic narcotic analgesic related to codeine, with multiple actions qualitatively similar to those of codeine; the most prominent of these involve the central nervous system and organs with smooth muscle components. The principal action of therapeutic value is analgesia.
This combination product also contains acetaminophen, a non-opiate, non-salicylate analgesic and antipyretic. This combination product contains caffeine as an analgesic adjuvant. Caffeine is also a central nervous system and cardiovascular stimulant.
- INDICATIONS AND USAGE:
-
CONTRAINDICATIONS:
This combination product is contraindicated in persons with hypersensitivity to dihydrocodeine, codeine, acetaminophen, caffeine, or any of the inactive components listed above, or any situation where opioids are contraindicated including significant respiratory depression (in unmonitored settings or in the absence of resuscitative equipment), acute or severe bronchial asthma or hypercapnia, and paralytic ileus.
-
WARNINGS:
Usage in Ambulatory Patients:
Dihydrocodeine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
Respiratory Depression:
Respiratory depression is the most dangerous acute reaction produced by opioid agonist preparations, although it is rarely severe with usual doses. Opioids decrease the respiratory rate, tidal volume, minute ventilation, and sensitivity to carbon dioxide. Respiratory depression occurs most frequently in elderly or debilitated patients, usually after large initial doses in nontolerant patients, or when opioids are given in conjunction with other agents that depress respiration. This combination product should be used with caution in patients with significant chronic obstructive pulmonary disease or corpulmonale and in patients with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or respiratory depression. In such patients, alternative non-opioid analgesics should be considered, and opioids should be admin- istered only under careful medical supervision at the lowest effective dose.
Head Injury:
This combination product should be used cautiously in the presence of head injury or increased intracranial pressure. The effects of opioids on pupillary response and consciousness may obscure neurologic signs of increases in intracranial pressure in patients with head injuries. The respiratory depressant effects including carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, intracranial lesions, or other causes of increased intracranial pressures.
Hypotensive Effect:
Dihydrocodeine, like all opioid analgesics, may cause hypotension in patients whose ability to maintain blood pressure has been compromised by a depleted blood volume or who receive concurrent therapy with drugs such as phenothiazines or other agents which compromise vasomotor tone.
Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets may produce orthostatic hypotension in ambulatory patients. This combination product should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Drug Dependence
Dihydrocodeine can produce drug dependence of the codeine type and has the potential of being abused (See DRUG ABUSE AND DEPENDENCE).
-
PRECAUTIONS:
General:
Selection of patients for treatment with Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets should be governed by the same principles that apply to the use of similar opioid/non-opioid fixed combination analgesics. As with any such opioid analgesic, the dosing regimen should be adjusted for each patient (see DOSAGE AND ADMINISTRATION). This combination product should be used with caution in elderly or debilitated patients or those with any of the following conditions: acute alcoholism; adrenocortical insufficiency (e.g., Addison's disease); asthma; central nervous system depression or coma; chronic obstructive pulmonary disease; decreased respiratory reserve (including emphysema, severe obesity, cor pulmonale, or kyphoscoliosis); delirium tremens; head injury; hypotension; increased intracranial pressure; myxedema or hypothyroidism; prostatic hypertrophy or urethral stricture; and toxic psychosis. The benefits and risks of using opioids in patients taking monoamine oxidase inhibitors and in those with a history of drug abuse should be carefully considered. The administration of an analgesic containing an opioid may obscure the diagnosis or clinical course in patients with acute abdominal conditions. This combination product may aggravate convulsions in patients with convulsive disorders and, like all opioids, may induce or aggravate seizures in some clinical settings. Acetaminophen is relatively non-toxic at therapeutic doses, but should be used with caution in patients with severe renal or hepatic disease. Care should be observed when using large doses of acetaminophen in malnourished patients or those with a history of chronic alcohol abuse because they may be more susceptible to hepatic damage similar to that observed with toxic overdosage.
Caffeine in high doses may produce central nervous system and cardiovascular stimulation and gastrointestinal irritation.
Drug-Drug Interactions:
Dihydrocodeine with Other Central Nervous System Depressants:
Patients receiving other opioid analgesics, sedatives or hypnotics, muscle relaxants, general anesthetics, centrally acting anti-emetics, phenothiazines or other tranquilizers, or alcohol concomitantly with this combination product may exhibit additive depressant effects on the central nervous system. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Dihydrocodeine with Monoamine Oxidase Inhibitors:
Dihydrocodeine, like all opioid analgesics, interacts with monoamine oxidase inhibitors causing central nervous system excitation and hypertension.
Dihydrocodeine with Mixed Agonist/Antagonist Opioid Analgesics:
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol and buprenorphine) may reduce the analgesic effect of this combination product.
Acetaminophen-Drug Interactions:
Chronic and excessive consumption of alcohol may increase the hepatotoxic risk of acetaminophen. The potential for hepatotoxicity with acetaminophen also may be increased in patients receiving anticonvulsants that induce hepatic microsomal enzymes (including phenytoin, barbiturates, and carbamazepine) or isoniazide. Chronic ingestion of large doses of acetaminophen may slightly potentiate the effects of warfarin- and indandione- derivative anticoagulants. Severe hypothermia is possible in patients receiving acetaminophen concomitantly with phenothiazines.
Caffeine-Drug Interactions:
Caffeine may enhance the cardiac inotropic effects of beta-adrenergic stimulating agents. Coadministration of caffeine and disulfiram may lead to a substantial decrease in caffeine clearance. Caffeine may increase the metabolism of other drugs such as phenobarbital and aspirin. Caffeine accumulation may occur when products or foods containing caffeine are consumed concomitantly with quinolones such as ciprofloxacin.
Information for Patients/Caregivers:
Patients receiving Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets should be given the following information:
- Patients should be advised that Acetaminophen, caffeine and dihydrocodeine bitartrate tablet may impair the mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Patients should be advised to report adverse experiences occurring during therapy.
- Patients should be advised not to adjust the dose of Acetaminophen, caffeine and dihydrocodeine bitartrate tablet without consulting the prescribing professional.
- Patients should not combine Acetaminophen, caffeine and dihydrocodeine bitartrate tablet with alcohol or other central nervous system depressants (sleep aids, tranquilizers) except by the orders of the prescribing physician, because additive effects may occur.
- Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
- Patients should be advised that Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets are a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
Pregnancy:
Teratogenic Effects – Pregnancy Category C.
Animal reproduction studies have not been conducted with Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets. It is also not known whether this combination product can cause fetal harm when administered to pregnant women or can affect reproduction capacity in males and females. This combination product should be given to pregnant women only if clearly needed, especially during the first trimester.
Labor and Delivery:
Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets are not recommended for use by women during and immediately before labor and delivery because oral opioids may cause respiratory depression in the newborn.
Nursing Mothers:
Dihydrocodeine bitartrate, acetaminophen and caffeine tablets are excreted in breast milk in small amounts, but the significance of their effects on nursing infants is not known. Because of the potential for serious adverse reactions in nursing infants from this combination product, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use:
Safety and effectiveness of Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets in pediatric patients have not been established.
Geriatric Use:
Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets should be given with caution to the elderly.
Hepatic Impairment:
Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets should be given with caution to patients with hepatic insufficiency. Since dihydrocodeine is metabolized by the liver and since acetaminophen potentially causes hepatotoxicity, the effects of this combination product should be monitored closely in such patients.
-
ADVERSE REACTIONS:
Dihydrocodeine:
The most frequently observed adverse reactions include light-headedness, dizziness, drowsiness, headache, fatigue, sedation, sweating, nausea, vomiting, constipation, pruritus, and skin reactions. With the exception of constipation, tolerance develops to most of these effects. Other reactions that have been observed with dihydrocodeine or other opioids include respiratory depression, orthostatic hypotension, cough suppression, confusion, diarrhea, miosis, abdominal pain, dry mouth, indigestion, anorexia, spasm of biliary tract, and urinary retention. Physical and psychological dependence are possibilities. Hypersensitivity reactions (including anaphylactoid reactions), hallucinations, vivid dreams, granulomatous interstitial nephritis, severe narcosis and acute renal failure have been reported rarely during dihydrocodeine administration.
Acetaminophen:
Acetaminophen in therapeutic doses rarely causes adverse reactions.
The most serious adverse reaction is hepatoxicity from overdosage (see OVERDOSAGE). Thrombocytopenia, leukopenia, pancytopenia, neutropenia, thrombocytopenic purpura, and agranulocytosis have been reported in patients receiving acetaminophen or p-aminophenol derivatives. Hypersensitivity reactions including urticarial or erythematous skin reactions, laryngeal edema, angioedema, or anaphylactoid reactions are rare.
Caffeine:
Adverse reactions associated with caffeine use include anxiety, anxiety neurosis, excitement, headaches, insomnia, irritability, lightheadedness, restlessness, tenseness, tremor, extrasystoles, palpitations, tachycardia, diarrhea, nausea, stomach pain, vomiting, diuresis, urticarcia, scintillating scotoma, and tinnitus.
-
DRUG ABUSE AND DEPENDENCE:
This combination product is subject to the provisions of the Controlled Substance Act, and has been placed in Schedule III.
Dihydrocodeine can produce drug dependence of the codeine type and therefore has the potential of being abused.
Like other opioid analgesics, dihydrocodeine may produce subjective effects other than analgesia (e.g., euphoria, relaxation), which may contribute to abuse by some patients. Psychological dependence, physical dependence, and tolerance may develop upon repeated administration of dihydrocodeine, and It should be prescribed and administered with the same degree of caution appropriate to the use of other oral opioid analgesic medications.
Symptoms of dihydrocodeine withdrawal consist of irritability, restlessness, insomnia, diaphoresis, anxiety and palpitations.
Prolonged, high intake of caffeine may produce tolerance and habituation. Physical signs of withdrawal, such as headaches, irritation, nervousness, anxiety, and dizziness may occur upon abrupt discontinuation.
-
OVERDOSAGE:
Following an acute overdosage with Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets, toxicity may result from the dihydrocodeine, acetaminophen, or, less likely, caffeine component. An overdose is a potentially lethal polydrug overdose situation, and consultation with a regional poison control center is recommended. A listing of the poison control centers can be found in standard references such as the Physician's Desk Reference®.
Signs and Symptoms and Laboratory Findings:
Toxicity from dihydrocodeine is typical of opioids and includes pinpoint pupils, respiratory depression, and loss of consciousness. Convulsions, cardiovascular collapse, and death may occur. A single case of acute rhabdomyolysis associated with an overdose of dihydrocodeine has been reported. With acetaminophen, dose-dependent potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may occur. Early symptoms of hepatotoxicity include nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours after ingestion. Acute caffeine poisoning may cause insomnia, restlessness, tremor, delirium, tachycardia, extrasystoles, and seizures.
Because overdose information on this combination product is limited, it is unclear which of the signs and symptoms of toxicity would manifest in any particular overdose situation.
Treatment:
Immediate treatment of an overdosage of Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets includes support of cardiorespiratory function and measures to reduce drug absorption. Vomiting should be induced with syrup of ipecac, if the patient is alert and has adequate laryngeal reflexes. Oral activated charcoal should follow. The first dose of charcoal should be accompanied by an appropriate cathartic. Gastric lavage may be necessary. Hypotension is usually hypovolemic and should be treated with fluids. Endotracheal intubation and artificial respiration may be necessary. Peritoneal or hemodialysis may be necessary. If hypoprothrombinemia occurs, Vitamin K should be administered.
A pure opioid antagonist, such as naloxone or nalmefene, is a specific antidote against respiratory depression which results from opioid overdose. Opioid antagonists should not be given in the absence of clinically significant respiratory or circulatory depression secondary to opioid overdose. They should be administered cautiously to persons who are known, or suspected to be, physically dependent on any opioid agonist including Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. The prescribing information for the specific opioid antagonist should be consulted for details of their proper use.
In adults and adolescents, regardless of the quantity of acetaminophen reported to have been ingested, acetylcysteine should be administered immediately if 24 hours or less have elapsed from the reported time of ingestion. It is not advisable to await the plasma concentration determination of acetaminophen before administering acetylcysteine. Serum liver enzyme levels should be measured. Therapy in children involves a similar treatment scheme; however, a regional Poison Control Center should be contacted. No specific antidote is available for caffeine. In addition to the supportive measures above, administration of demulcents such as aluminum hydroxide gel may diminish gastrointestinal irritation. Seizures may be treated with intravenous diazepam or a barbiturate.
-
DOSAGE AND ADMINISTRATION
The usual adult dosage is one (1) Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets orally every four (4) hours, as needed. Dosage should be adjusted according to the severity of the pain and the response of the patient. No more than one (1) tablet should be taken in a 4-hour period. No more than five (5) doses, or five (5) tablets should be taken in a 24-hour period.
-
HOW SUPPLIED:
Acetaminophen, caffeine, and dihydrocodeine bitartrate tablets, containing acetaminophen 712.8 mg, caffeine 60 mg and dihydrocodeine* bitartrate 32 mg (*Warning: May be habit-forming). Tablets are white, oval-shaped single scored and are debossed “Boca” on one side and “611” on the other side; are supplied in
Bottles of 30
NDC 54868-5900-0
Store at 20°C to 25°C (68°F to 77°F). [see USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight, light-resistant container with a child-resistant closure.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Rx Only
Manufactured for:
Boca Pharmacal, Inc.
Coral Springs, FL 33065
www.bocapharmacal.comRev. 06/09
Physician’s Desk Reference® is the registered trademark of Thomson Healthcare, Inc.
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, OK 74146
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIHYDROCODEINE BITARTRATE, ACETAMINOPHEN AND CAFFEINE
dihydrocodeine bitartrate, acetaminophen and caffeine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5900(NDC:64376-611) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIHYDROCODEINE BITARTRATE (UNII: 8LXS95BSA9) (DIHYDROCODEINE - UNII:N9I9HDB855) DIHYDROCODEINE BITARTRATE 32 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 712.8 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 60 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score 2 pieces Shape OVAL Size 19mm Flavor Imprint Code Boca;611 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5900-0 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040701 05/20/2008 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel, repack