QUIP- sodium monofluorophosphate paste, dentifrice
Quip NYC Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
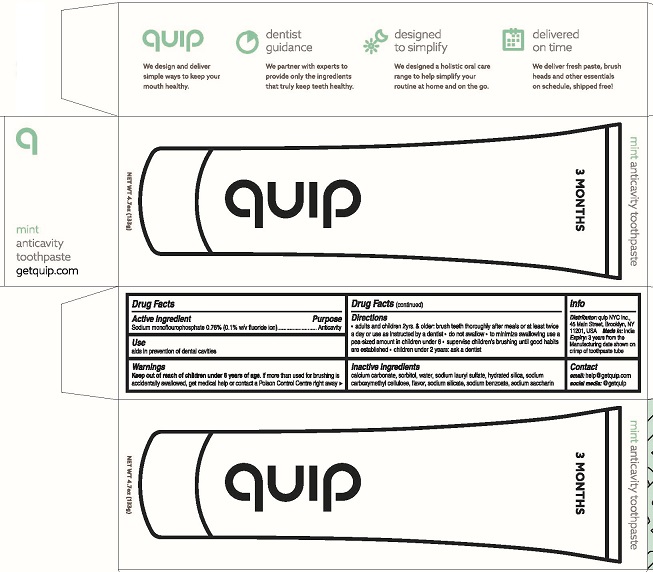
Quip Anticavity Toothpaste
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Centre right away.
Directions
- adults and children 2yrs. & older brush teeth thoroughly after meals or at least twice a day or use as instructed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6 years
- supervise children's brushing until good habits are established
- children under 2 years: ask a dentist
Inactive ingredients
calcium carbonate, sorbitol, water, sodium lauryl sulfate, hydrated silica, sodium carboxymethyl cellulose, flavor, sodium silicate, sodium benzoate, sodium saycharin
Distributor: quip NYC Inc.,
45 Main Street
Brooklyn, NY 11201, USA
Made in:India
Expiry: 3 years from the Manufacturing date shown on crimp of tube
quip
We design and deliver simple ways to keep your mouth healthy.
dentist guidance
We partner with experts to provide only the ingredients that truly keep teeth healthy.
designed to simplify
We designed a holistic oral care range to help simplify your routine at home or on the go.
delivered on time
We deliver fresh paste, brush heads and other essentials on schedule, shipped free!
| QUIP
sodium monofluorophosphate paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Quip NYC Inc. (079453380) |