WALGREENSTRIPLE ANTIBIOTIC- bacitracin, neomycin, polymyxin b ointment
Walgreen Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Package Labeling
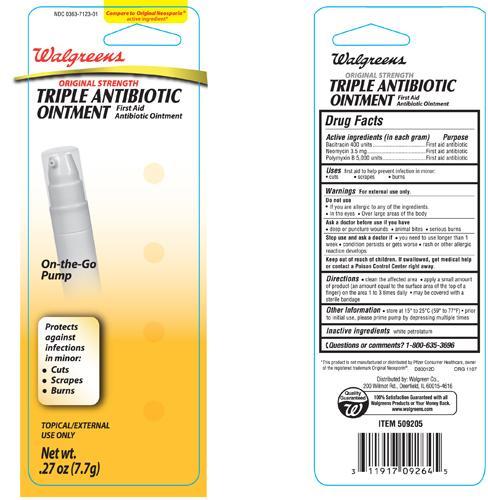
Drug Facts
Active Ingredients (in each gram)
Bacitracin 400 units
Neomycin 3.5 mg
Polymyxin B 5,000 units
Warnings
For external use only.
Do not use
• if you are allergic to any of the ingredients
• in the eyes
• over large areas of the body
Directions
• clean the affected area • apply a small amount of product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily • may be covered with a sterile bandage
| WALGREENSTRIPLE ANTIBIOTIC
bacitracin, neomycin, polymyxin b ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
| Registrant - Wisconsin Pharmacal Company (800873986) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wisconsin Pharmacal Company | 800873986 | manufacture(0363-7123) | |
Revised: 12/2017
Document Id: 5f8ad9e0-ddbc-1c24-e053-2a91aa0ad070
Set id: f32137d3-9616-4528-a3e2-62a1436511ca
Version: 5
Effective Time: 20171204
Walgreen Company