Label: ALLERGY RELIEF NON DROWSY- loratadine tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 52904-430-01 - Packager: Select Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 15, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
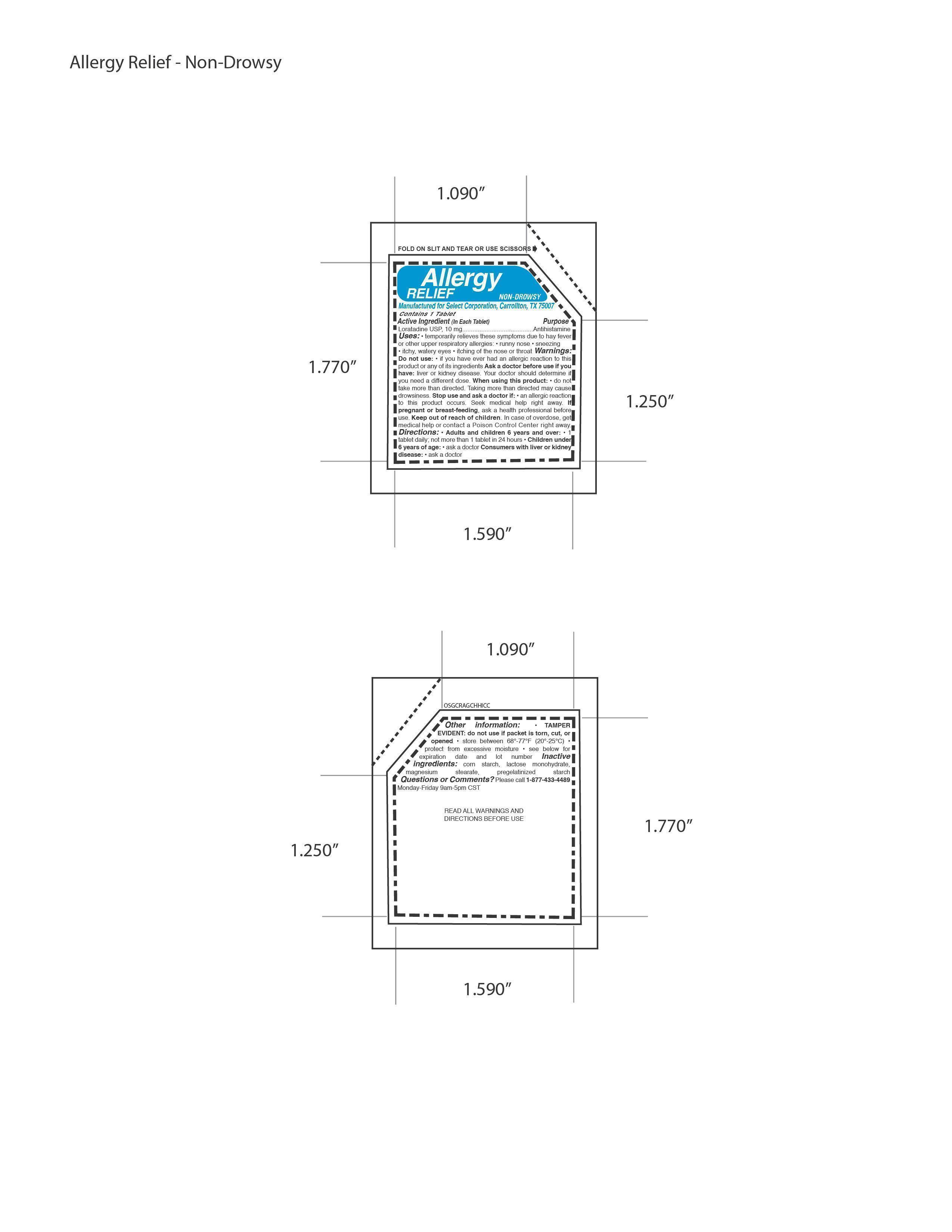
WARNINGS
Warnings:
Do not use: • if you have ever had an allergic reaction to this
product or any of its ingredients Ask a doctor before use if you
have: liver or kidney disease. Your doctor should determine if
you need a different dose. When using this product: • do not
take more than directed. Taking more than directed may cause
drowsiness. Stop use and ask a doctor if: • an allergic reaction
to this product occurs. Seek medical help right away. - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF NON DROWSY
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52904-430 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) LACTOSE (UNII: J2B2A4N98G) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (snow white) Score no score Shape ROUND (RX526) Size 6mm Flavor Imprint Code RX526 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52904-430-01 1 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077153 10/15/2012 Labeler - Select Corporation (053805599) Registrant - Select Corporation (053805599)