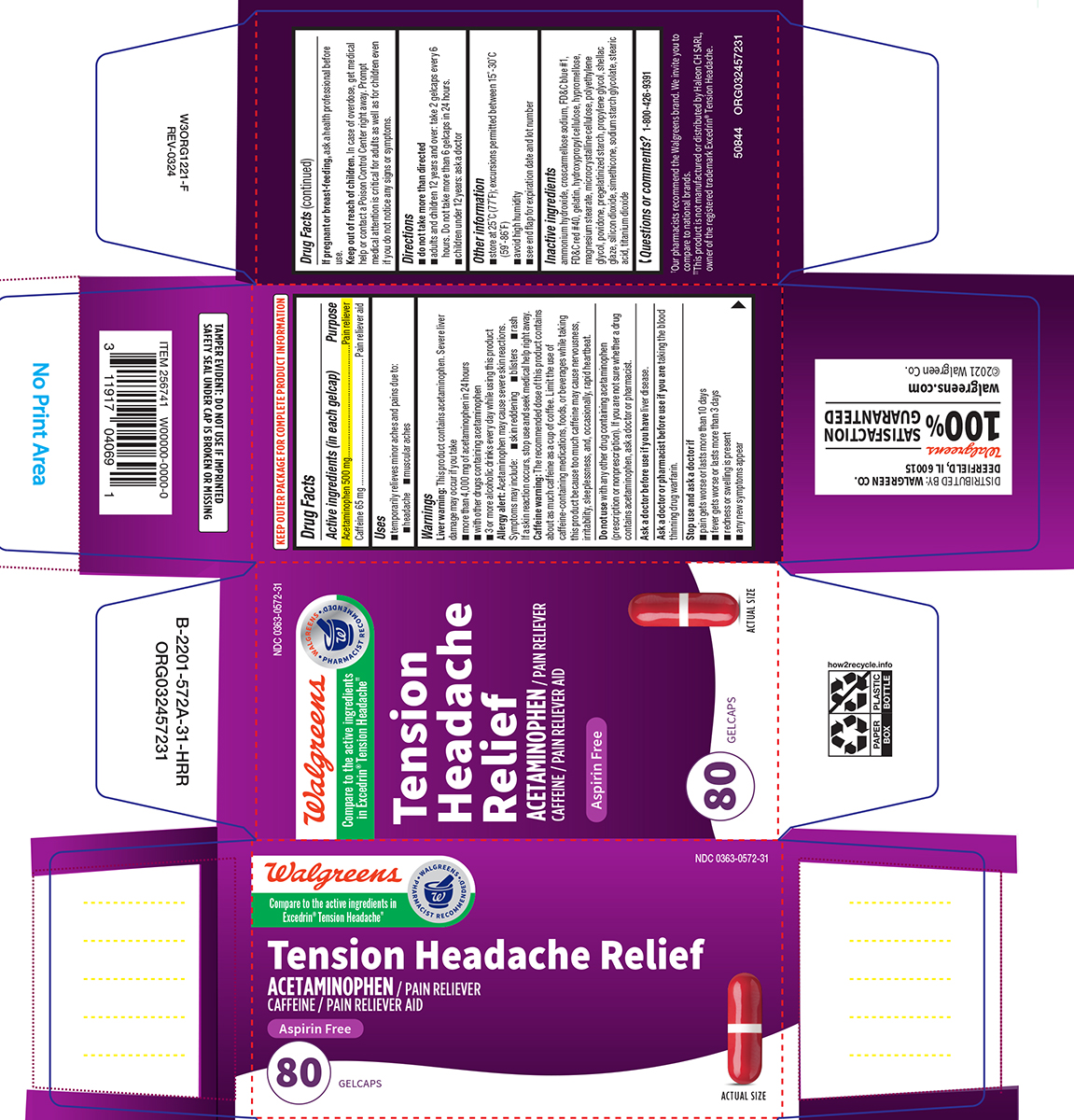
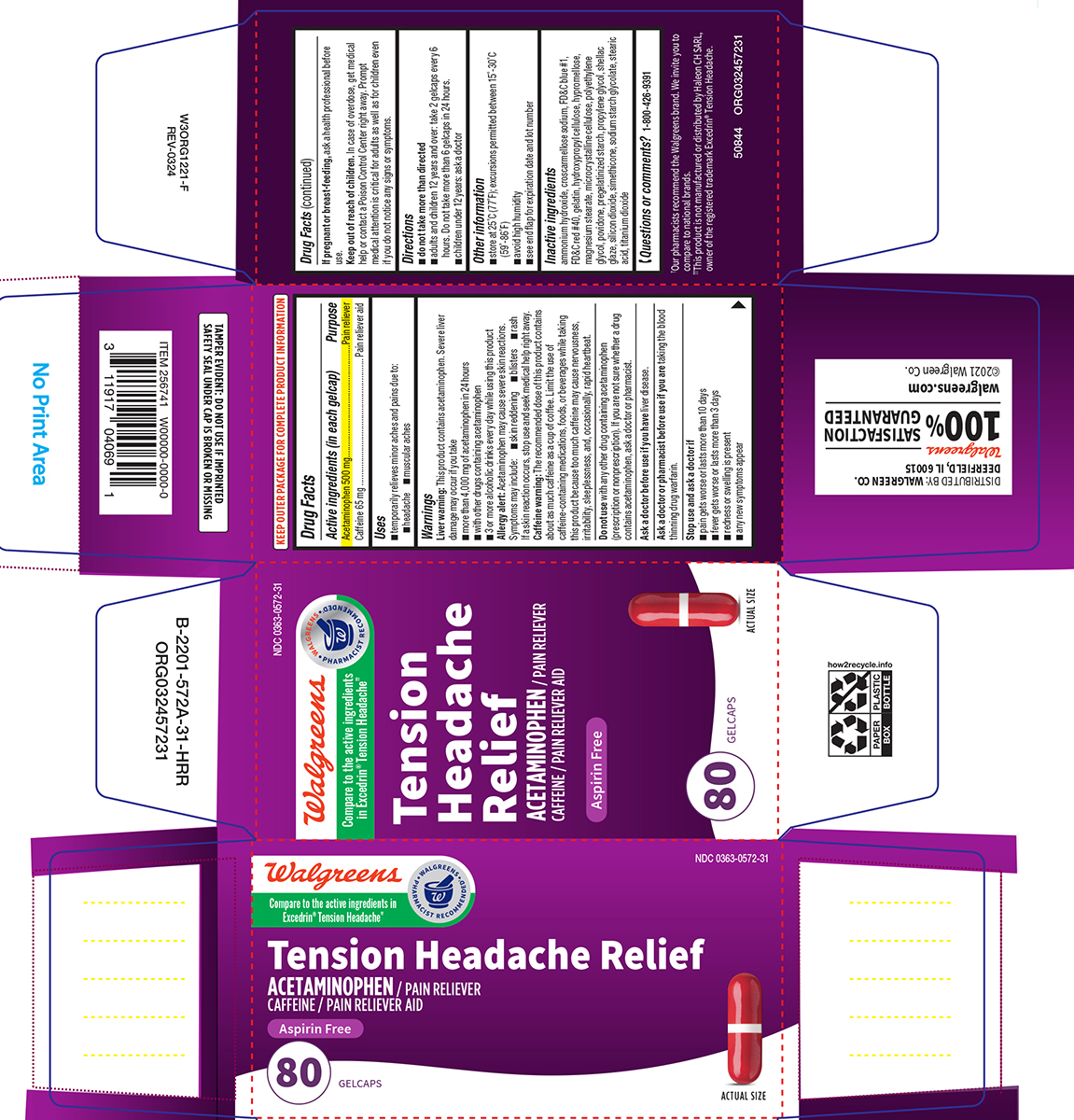
Label: TENSION HEADACHE RELIEF- acetaminophen, caffeine tablet, coated
- NDC Code(s): 0363-0572-09, 0363-0572-31
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each gelcap)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat.
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- Directions
- Other information
-
Inactive ingredients
ammonium hydroxide, croscarmellose sodium, FD&C blue #1, FD&C red #40, gelatin, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, silicon dioxide, simethicone, sodium starch glycolate, stearic acid, titanium dioxide
- Questions or comments?
-
Principal Display Panel
Walgreens
Compare to the active ingredients
in Excedrin® Tension Headache††• WALGREENS •
PHARMACIST RECOMMENDED†NDC 0363-0572-31
Tension
Headache
ReliefACETAMINOPHEN / PAIN RELIEVER
CAFFEINE / PAIN RELIEVER AIDASPIRIN FREE
80 GELCAPS
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING†Our pharmacists recommend the Walgreens brand. We invite you
to compare to national brands.
††This product is not manufactured or distributed by Haleon CH SARL,
owner of the registered trademark Excedrin® Tension HeadacheDISTRIBUTED BY: WALGREEN CO.
DEERFIELD, IL 60015
Walgreens
100% SATISFACTION
GUARANTEED
walgreens.com ©2021 Walgreen Co.50844 ORG032457231
Walgreens 44-572
-
INGREDIENTS AND APPEARANCE
TENSION HEADACHE RELIEF
acetaminophen, caffeine tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0572 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape OVAL Size 20mm Flavor Imprint Code L;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0572-31 1 in 1 CARTON 02/09/2009 1 80 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:0363-0572-09 1 in 1 CARTON 02/09/2009 07/08/2018 2 20 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 02/09/2009 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 manufacture(0363-0572) , pack(0363-0572) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0363-0572) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(0363-0572) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0363-0572) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0363-0572)