METOCLOPRAMIDE HYDROCHLORIDE- metoclopramide hydrochloride tablet
REMEDYREPACK INC.
----------
BOXED WARNING
WARNING
TARDIVE DYSKINESIA
Treatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing tardive dyskinesia increases with duration of treatment and total cumulative dose.
Metoclopramide therapy should be discontinued in patients who develop signs or symptoms of tardive dyskinesia. There is no known treatment for tardive dyskinesia. In some patients, symptoms may lessen or resolve after metoclopramide treatment is stopped.
Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing tardive dyskinesia.
WARNINGS
DESCRIPTION
DESCRIPTION
For oral administration, Metoclopramide tablets, USP 10 mg are white to off white, capsule shaped, biconvex tablets with "RF11" embossed on one side and score line on the other side.
Each tablet contains:
10 mg of metoclopramide base as metoclopramide hydrochloride USP
Inactive Ingredients
Microcrystalline Cellulose, Corn starch, Pregelatinized starch, Colloidal silicon dioxide and Stearic acid.
Metoclopramide tablets, USP 5 mg are white to off white, oval shaped, biconvex tablets with "RF10" embossed on one side and plain on the other side.
Each tablet contains:
5 mg of metoclopramide base as metoclopramide hydrochloride USP
Inactive Ingredients
Microcrystalline cellulose, Corn starch, Pregelatinized starch, Colloidal silicon dioxide and Stearic acid.
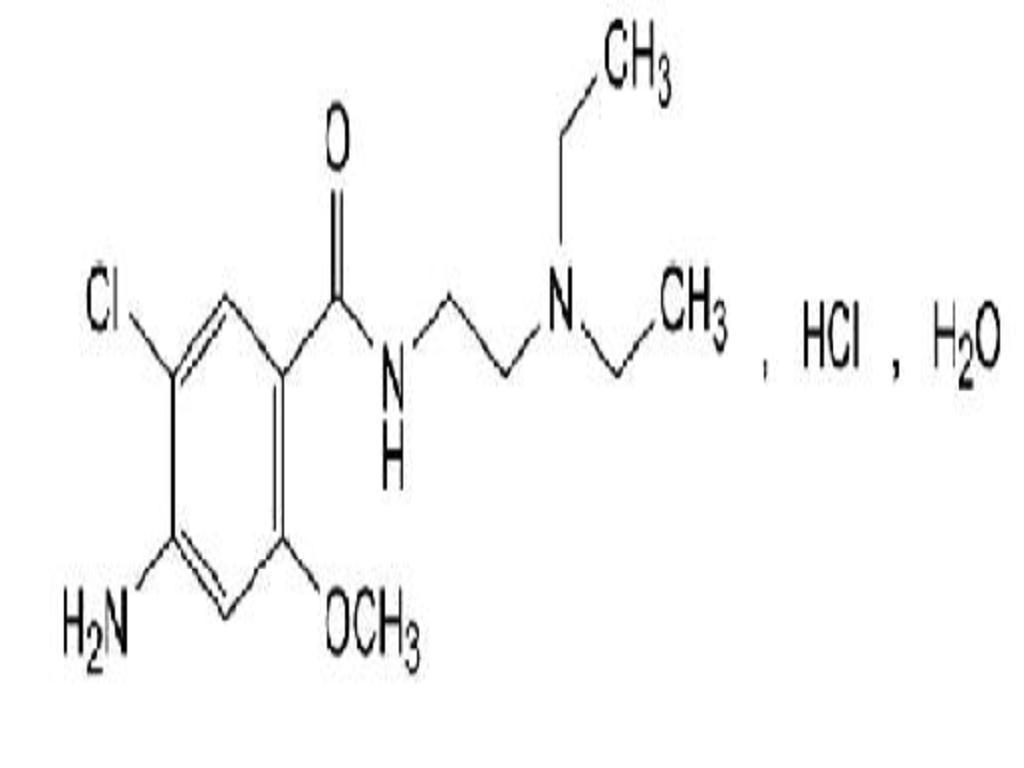
Metoclopramide hydrochloride is a white crystalline, odorless substance, freely soluble in water. Chemically, it is 4-amino-5-chloro-N-[2-(diethylamino) ethyl]-2-methoxy benzamide monohydrochloride monohydrate. Its molecular formula is C14H22CIN3O2HCIH2O. Its molecular weight is 354.3.
CLINICAL PHARMACOLOGY
Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. Its mode of action is unclear. It seems to sensitize tissues to the action of acetylcholine. The effect of metoclopramide on motility is not dependent on intact vagal innervation, but it can be abolished by anticholinergic drugs.
Metoclopramide increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder.
In patients with gastroesophageal reflux and low LESP (lower esophageal sphincter pressure), single oral doses of metoclopramide produce dose-related increases in LESP. Effects begin at about 5 mg and increase through 20 mg (the largest dose tested). The increase in LESP from a 5 mg dose lasts about 45 minutes and that of 20 mg lasts between 2 and 3 hours. Increased rate of stomach emptying has been observed with single oral doses of 10 mg.
The antiemetic properties of metoclopramide appear to be a result of its antagonism of central and peripheral dopamine receptors. Dopamine produces nausea and vomiting by stimulation of the medullary chemoreceptor trigger zone (CTZ), and metoclopramide blocks stimulation of the CTZ by agents like l-dopa or apomorphine, which are known to increase dopamine levels or to possess dopamine-like effects. Metoclopramide also abolishes the slowing of gastric emptying caused by apomorphine.
Like the phenothiazines and related drugs, which are also dopamine antagonists, metoclopramide produces sedation and may produce extrapyramidal reactions, although these are comparatively rare (see
WARNINGS
). Metoclopramide inhibits the central and peripheral effects of apomorphine, induces release of prolactin and causes a transient increase in circulating aldosterone levels, which may be associated with transient fluid retention.
The onset of pharmacological action of metoclopramide is 1 to 3 minutes following an intravenous dose, 10 to 15 minutes following intramuscular administration, and 30 to 60 minutes following an oral dose; pharmacological effects persist for 1 to 2 hours.
PHARMACOKINETICS
Metoclopramide is rapidly and well absorbed. Relative to an intravenous dose of 20 mg, the absolute oral bioavailability of metoclopramide is 80%15.5% as demonstrated in a crossover study of 18 subjects. Peak plasma concentrations occur at about 1 to 2 hr after a single oral dose. Similar time to peak is observed after individual doses at steady state.
Approximately 85% of the radioactivity of an orally administered dose appears in the urine within 72 hr. Of the 85% eliminated in the urine, about half is present as free or conjugated metoclopramide.
The drug is not extensively bound to plasma proteins (about 30%). The whole body volume of distribution is high (about 3.5 L/kg) which suggests extensive distribution of drug to the tissues.
Renal impairment affects the clearance of metoclopramide. In a study with patients with varying degrees of renal impairment, a reduction in creatinine clearance was correlated with a reduction in plasma clearance, renal clearance, non-renal clearance, and increase in elimination half-life. The kinetics of metoclopramide in the presence of renal impairment remained linear however. The reduction in clearance as a result of renal impairment suggests that adjustment downward of maintenance dosage should be done to avoid drug accumulation.
Adult Pharmacokinetic Data
ParameterValue
Vd (L/kg)~ 3.5Plasma Protein Binding~ 30%t1/2 (hr)5 to 6Oral Bioavailability80%In pediatric patients, the pharmacodynamics of metoclopramide following oral and intravenous administration are highly variable and a concentration-effect relationship has not been established.
There are insufficient reliable data to conclude whether the pharmacokinetics of metoclopramide in adults and the pediatric population are similar. Although there are insufficient data to support the efficacy of metoclopramide in pediatric patients with symptomatic gastroesophageal reflux (GER) or cancer chemotherapy-related nausea and vomiting, its pharmacokinetics have been studied in these patient populations.
In an open-label study, six pediatric patients (age range, 3.5 weeks to 5.4 months) with GER received metoclopramide 0.15 mg/kg oral solution every 6 hours for 10 doses. The mean peak plasma concentration of metoclopramide after the tenth dose was 2-fold (56.8higher compared to that observed after the first dose (29indicating drug accumulation with repeated dosing. After the tenth dose, the mean time to reach peak concentrations (2.2 hr), half-life (4.1 hr), clearance (0.67 L/h/kg), and volume of distribution (4.4 L/kg) of metoclopramide were similar to those observed after the first dose. In the youngest patient (age, 3.5 weeks), metoclopramide half-life after the first and the tenth dose (23.1 and 10.3 hr, respectively) was significantly longer compared to other infants due to reduced clearance. This may be attributed to immature hepatic and renal systems at birth.
In another study, nine pediatric cancer patients (age range, 1 to 9 yr) received 4 to 5 intravenous infusions (over 30 minutes) of metoclopramide at a dose of 2 mg/kg to control emesis. After the last dose, the peak serum concentrations of metoclopramide ranged from 1060 to 5680The mean elimination half-life, clearance, and volume of distribution of metoclopramide were 4.5 hr (range, 2.0 to 12.5 hr), 0.37 L/h/kg (range, 0.10 to 1.24 L/h/kg), and 1.93 L/kg (range, 0.95 to 5.50 L/kg), respectively.
INDICATIONS & USAGE
The use of metoclopramide tablets is recommended for adults only. Therapy should not exceed 12 weeks in duration.
Symptomatic Gastroesophageal Reflux
Metoclopramide tablets are indicated as short-term (4 to 12 weeks) therapy for adults with symptomatic, documented gastroesophageal reflux who fail to respond to conventional therapy.
The principal effect of metoclopramide is on symptoms of postprandial and daytime heartburn with less observed effect on nocturnal symptoms. If symptoms are confined to particular situations, such as following the evening meal, use of metoclopramide as single doses prior to the provocative situation should be considered, rather than using the drug throughout the day. Healing of esophageal ulcers and erosions has been endoscopically demonstrated at the end of a 12-week trial using doses of 15 mg q.i.d. As there is no documented correlation between symptoms and healing of esophageal lesions, patients with documented lesions should be monitored endoscopically.
Diabetic Gastroparesis (Diabetic Gastric Stasis)
Metoclopramide tablets, are indicated for the relief of symptoms associated with acute and recurrent diabetic gastric stasis. The usual manifestations of delayed gastric emptying (e.g., nausea, vomiting, heartburn, persistent fullness after meals, and anorexia) appear to respond to metoclopramide tablets within different time intervals. Significant relief of nausea occurs early and continues to improve over a three-week period. Relief of vomiting and anorexia may precede the relief of abdominal fullness by one week or more.
CONTRAINDICATIONS
Metoclopramide should not be used whenever stimulation of gastrointestinal motility might be dangerous, e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation.
Metoclopramide is contraindicated in patients with pheochromocytoma because the drug may cause a hypertensive crisis, probably due to release of catecholamines from the tumor. Such hypertensive crises may be controlled by phentolamine.
Metoclopramide is contraindicated in patients with known sensitivity or intolerance to the drug.
Metoclopramide should not be used in epileptics or patients receiving other drugs which are likely to cause extrapyramidal reactions, since the frequency and severity of seizures or extrapyramidal reactions may be increased.
WARNINGS
Mental depression has occurred in patients with and without prior history of depression. Symptoms have ranged from mild to severe and have included suicidal ideation and suicide. Metoclopramide should be given to patients with a prior history of depression only if the expected benefits outweigh the potential risks.
Extrapyramidal symptoms, manifested primarily as acute dystonic reactions, occur in approximately 1 in 500 patients treated with the usual adult dosages of 30 to 40 mg/day of metoclopramide.These usually are seen during the first 24 to 48 hours of treatment with metoclopramide, occur more frequently in pediatric patients and adult patients less than 30 years of age and are even more frequent at higher doses. These symptoms may include involuntary movements of limbs and facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, or dystonic reactions resembling tetanus. Rarely, dystonic reactions may present as stridor and dyspnea, possibly due to laryngospasm. If these symptoms should occur, inject 50 mg diphenhydramine hydrochloride intramuscularly, and they usually will subside. Benztropine mesylate, 1 to 2 mg intramuscularly, may also be used to reverse these reactions.
Parkinsonian-like symptoms have occurred, more commonly within the first 6 months after beginning treatment with metoclopramide, but occasionally after longer periods. These symptoms generally subside within 2 to 3 months following discontinuance of metoclopramide. Patients with preexisting Parkinson's disease should be given metoclopramide cautiously, if at all, since such patients may experience exacerbation of parkinsonian symptoms when taking metoclopramide.
Tardive Dyskinesia
(see Boxed Warnings)
Treatment with metoclopramide can cause tardive dyskinesia (TD), a potentially irreversible and disfiguring disorder characterized by involuntary movements of the face, tongue, or extremities. Although the risk of TD with metoclopramide has not been extensively studied, one published study reported a TD prevalence of 20% among patients treated for at least 12 weeks. Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing TD.
Although the risk of developing TD in the general population may be increased among the elderly, women, and diabetics, it is not possible to predict which patients will develop metoclopramide-induced TD. Both the risk of developing TD and the likelihood that TD will become irreversible increase with duration of treatment and total cumulative dose.
Metoclopramide should be discontinued in patients who develop signs or symptoms of TD. There is no known effective treatment for established cases of TD, although in some patients, TD may remit, partially or completely, within several weeks to months after metoclopramide is withdrawn.
Metoclopramide itself may suppress, or partially suppress, the signs of TD, thereby masking the underlying disease process. The effect of this symptomatic suppression upon the long-term course of TD is unknown. Therefore, metoclopramide should not be used for the symptomatic control of TD.
There have been rare reports of an uncommon but potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) associated with metoclopramide. Clinical manifestations of NMS include hyperthermia, muscle rigidity, altered consciousness, and evidence of autonomic instablility (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac arrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, malignant hyperthermia, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of metoclopramide and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. Bromocriptine and dantroline sodium have been used in treatment of NMS, but their effectiveness have not been established (seeADVERSE REACTIONS
).
PRECAUTIONS
General
Because metoclopramide produces a transient increase in plasma aldosterone, certain patients, especially those with cirrhosis or congestive heart failure, may be at risk of developing fluid retention and volume overload. If these side effects occur at any time during metoclopramide therapy, the drug should be discontinued.
Adverse reactions, especially those involving the nervous system, may occur after stopping the use of metoclopramide tablets. A small number of patients may experience a withdrawal period after stopping metoclopramide tablets that could include dizziness, nervousness and/or headaches.
INFORMATION FOR PATIENTS
The use of metoclopramide tablets is recommended for adults only. Metoclopramide may impair the mental and/or physical abilities required for the performance of hazardous tasks such as operating machinery or driving a motor vehicle. The ambulatory patient should be cautioned accordingly.
For additional information, patients should be instructed to see the Medication Guide for Metoclopramide tablets.
DRUG INTERACTIONS
The effects of metoclopramide on gastrointestinal motility are antagonized by anticholinergic drugs and narcotic analgesics. Additive sedative effects can occur when metoclopramide is given with alcohol, sedatives, hypnotics, narcotics, or tranquilizers.
The finding that metoclopramide releases catecholamines in patients with essential hypertension suggests that it should be used cautiously, if at all, in patients receiving monoamine oxidase inhibitors.
Absorption of drugs from the stomach may be diminished (e.g., digoxin) by metoclopramide, whereas the rate and/or extent of absorption of drugs from the small bowel may be increased (e.g., acetaminophen, tetracycline, levodopa, ethanol, cyclosporine).
Gastroparesis (gastric stasis) may be responsible for poor diabetic control in some patients. Exogenously administered insulin may begin to act before food has left the stomach and lead to hypoglycemia. Because the action of metoclopramide will influence the delivery of food to the intestines and thus the rate of absorption, insulin dosage or timing of dosage may require adjustment.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
An Ames mutagenicity test performed on metoclopramide was negative.
PREGNANCY
Pregnancy Category B
Reproduction studies performed in rats, mice and rabbits by the I.V., I.M., S.C., and oral routes at maximum levels ranging from 12 to 250 times the human dose have demonstrated no impairment of fertility or significant harm to the fetus due to metoclopramide. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
NURSING MOTHERS
Metoclopramide is excreted in human milk. Caution should be exercised when metoclopramide is administered to a nursing mother.
PEDIATRIC USE
Safety and effectiveness in pediatric patients have not been established (see
OVERDOSAGE
).
Care should be exercised in administering metoclopramide to neonates since prolonged clearance may produce excessive serum concentrations (see
CLINICAL PHARMACOLOGYPharmacokinetics
). In addition, neonates have reduced levels of NADH-cytochrome b5 reductase which, in combination with the aforementioned pharmacokinetic factors, make neonates more susceptible to methemoglobinemia (see
OVERDOSAGE
).
The safety profile of metoclopramide in adults cannot be extrapolated to pediatric patients. Dystonias and other extrapyramidal reactions associated with metoclopramide are more common in the pediatric population than in adults. (See
WARNINGS
and
ADVERSE REACTIONSExtrapyramidal Reactions
.)
GERIATRIC USE
Clinical studies of metoclopramide tablets did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects.
The risk of developing parkinsonianlike side effects increases with ascending dose. Geriatric patients should receive the lowest dose of metoclopramide tablets that is effective. If parkinsonian like symptoms develop in a geriatric patient receiving metoclopramide tablets, metoclopramide tablets, should generally be discontinued before initiating any specific anti-parkinsonian agents(see
WARNINGS
and
DOSAGE AND ADMINISTRATIONFor the Relief of Symptomatic Gastroesophageal Reflux
).
The elderly may be at greater risk for tardive dyskinesia (see
WARNINGSTardive Dyskinesia
).
Sedation has been reported in metoclopramide tablets users. Sedation may cause confusion and manifest as over-sedation in the elderly (see
CLINICAL PHARMACOLOGY
,
PRECAUTIONS-Information for Patients
and
ADVERSE REACTIONSCNS Effects
).
Metoclopramide tablets is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function (see
DOSAGE AND ADMINISTRATION - Use in Patients with Renal or Hepatic Impairment).
For these reasons, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased renal function, concomitant disease, or other drug therapy in the elderly (see
DOSAGE AND ADMINISTRATIONFor the Relief of Symptomatic Gastroesophageal Reflux
and
Use in Patients with Renal or Hepatic Impairment
).
Other Special Populations
Patients with NADH-cytochrome b5 reductase deficiency are at an increased risk of developing methemoglobinemia and/or sulfhemoglobinemia when metolcopramide is administered. In patients with G6PD deficiency who experience metoclopramide-induced methemoglobinemia, methylene blue treated is not recommended (see
OVERDOSAGE
).
OVERDOSAGE
Symptoms of overdosage may include drowsiness, disorientation and extrapyramidal reactions. Anticholinergic or antiparkinson drugs or antihistamines with anticholinergic properties may be helpful in controlling the extrapyramidal reactions. Symptoms are self-limiting and usually disappear within 24 hours.
Hemodialysis removes relatively little metoclopramide, probably because of the small amount of the drug in blood relative to tissues. Similarly, continuous ambulatory peritoneal dialysis does not remove significant amounts of drug. It is unlikely that dosage would need to be adjusted to compensate for losses through dialysis. Dialysis is not likely to be an effective method of drug removal in overdose situations.
Unintentional overdose due to misadministration has been reported in infants and children with the use of metoclopramide oral solution. While there was no consistent pattern to the reports associated with these overdoses, events included seizures, extra pyramidal reactions, and lethargy.
Methemoglobinemia has occurred in premature and full-term neonates who were given overdoses of metoclopramide (1 to 4 mg/kg/day orally, intramuscularly or intravenously for 1 to 3 or more days). Methemoglobinemia can be reversed by the intravenous administration of methylene blue. However, methylene blue may cause hemolytic anemia in patients with G6PD deficiency, which may be fatal (see
PRECAUTIONSOther Special Populations
).
DOSAGE & ADMINISTRATION
Therapy with metoclopramide tablets should not exceed 12 weeks in duration.
For the Relief of Symptomatic Gastroesophageal Reflux
Administer from 10 mg to 15 mg metoclopramide tablets, USP orally up to q.i.d. 30 minutes before each meal and at bedtime, depending upon symptoms being treated and clinical response (see
CLINICAL PHARMACOLOGY
and
INDICATIONS AND USAGE
). If symptoms occur only intermittently or at specific times of the day, use of metoclopramide in single doses up to 20 mg prior to the provoking situation may be preferred rather than continuous treatment. Occasionally, patients (such as elderly patients) who are more sensitive to the therapeutic or adverse effects of metoclopramide will require only 5 mg per dose.
Experience with esophageal erosions and ulcerations is limited, but healing has thus far been documented in one controlled trial using q.i.d. therapy at 15 mg/dose, and this regimen should be used when lesions are present, so long as it is tolerated (see
ADVERSE REACTIONS
). Because of the poor correlation between symptoms and endoscopic appearance of the esophagus, therapy directed at esophageal lesions is best guided by endoscopic evaluation.
Therapy longer than 12 weeks has not been evaluated and cannot be recommended.
For the Relief of Symptoms Associated with Diabetic Gastroparesis (Diabetic Gastric Stasis)
Administer 10 mg of metoclopramide 30 minutes before each meal and at bedtime for two to eight weeks, depending upon response and the likelihood of continued well-being upon drug discontinuation.
The initial route of administration should be determined by the severity of the presenting symptoms. If only the earliest manifestations of diabetic gastric stasis are present, oral administration of metoclopramide tablets may be initiated. However, if severe symptoms are present, therapy should begin with metoclopramide injection (consult labeling of the injection prior to initiating parenteral administration).
Administration of metoclopramide injection up to 10 days may be required before symptoms subside, at which time oral administration may be instituted. Since diabetic gastric stasis is frequently recurrent, metoclopramide tablets therapy should be reinstituted at the earliest manifestation.
Use in Patients with Renal or Hepatic Impairment
Since metoclopramide is excreted principally through the kidneys, in those patients whose creatinine clearance is below 40 mL/min, therapy should be initiated at approximately one-half the recommended dosage. Depending upon clinical efficacy and safety considerations, the dosage may be increased or decreased as appropriate.
OVERDOSAGE
section for information regarding dialysis.
Metoclopramide undergoes minimal hepatic metabolism, except for simple conjugation. Its safe use has been described in patients with advanced liver disease whose renal function was normal.
HOW SUPPLIED
Each white to off white, capsule shaped biconvex metoclopramide tablets, USP with "RF11" embossed on one side & score line on the other side contains 10 mg of metoclopramide base as metoclopramide hydrochloride USP. Available in:
Bottle of 30 tablets NDC 63304-846-30
Bottle of 100 tablets NDC 63304-846-01
Bottle of 500 tablets NDC 63304-846-05
Bottle of 1000 tablets NDC 63304-846-10
Each white to off white, oval shaped biconvex tablets metoclopramide tablets, USP with "RF10" embossed on one side and plain on the other side contains 5 mg of metoclopramide base as metoclopramide hydrochloride USP. Available in
Bottles of 30 tablets NDC 63304-845-30
Bottle of 100 tablets NDC 63304-845-01
Bottle of 500 tablets NDC 63304-845-05
Bottle of 1000 tablets NDC 63304-845-10
STORAGE AND HANDLING
Store between 20and 25(68and 77[See USP Controlled Room Temperature].
Dispense tablets in tight, light-resistant container.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Manufactured for:
Ranbaxy Pharmaceuticals Inc.
Jacksonville, FL 32257 USA.
by: Ipca Laboratories Limited
48, Kandivli Ind. Estate,
Mumbai 400 067, India.
To obtain the Medication Guide online please visit www.ranbaxyusa.com.
October 2009
| METOCLOPRAMIDE HYDROCHLORIDE
metoclopramide hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |