PEDIACARE CHILDRENS ALLERGY- diphenhydramine hcl liquid
Prestige Brands Holdings, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
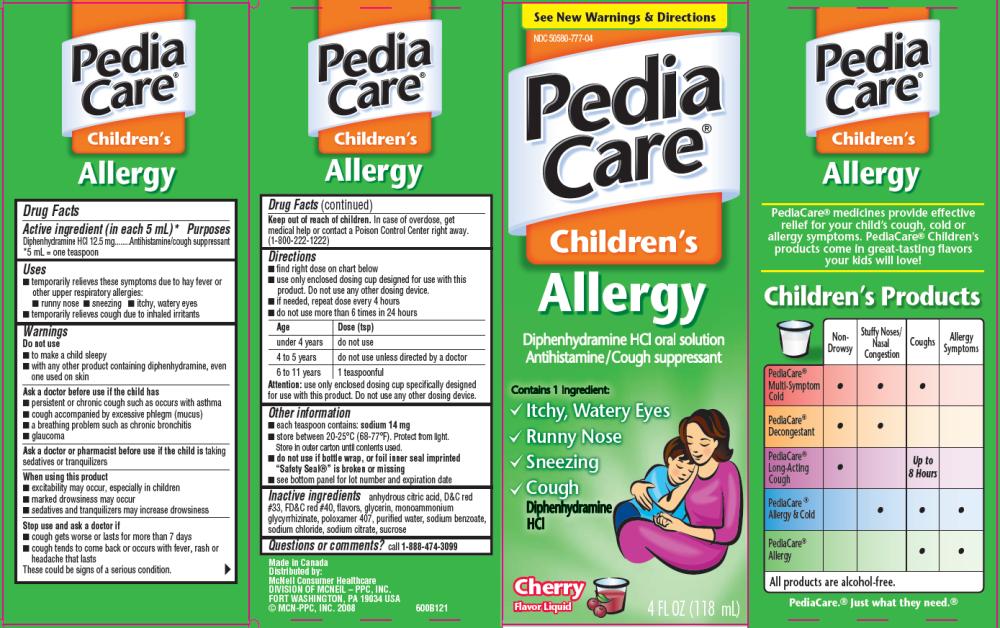
PediaCare Children’s Allergy Cherry Flavor
Drug Facts
Active ingredient
(in each 5 mL)*
Diphenhydramine HCI 12.5 mg
Purpose
Antihistamine/cough suppressant
*5 mL = one teaspoon
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- temporarily relieves cough due to inhaled irritants
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use
if the child has
- persistent or chronic cough such as occurs with asthma
- cough accompanied by excessive phlegm (mucus)
- a breathing problem such as chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use
if the child is taking sedatives or tranquilizers
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- sedatives and tranquilizers may increase drowsiness
Stop use and ask a doctor if
- cough gets worse or lasts for more than 7 days
- cough tends to come back or occurs with fever, rash or headache that lasts
These could be signs of a serious condition.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- find right dose on chart below
- use only enclosed dosing cup designed for use with this product. Do not use any other dosing device.
- if needed, repeat dose every 4 hours
- do not use more than 6 times in 24 hours
| Age (yr) | Dose (tsp) |
| Under 4 years | Do not use |
| 4 to 5 years | Do not use unless directed by a doctor |
| 6 to 11 years | 1 teaspoonful |
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
Other information
- each teaspoon contains: sodium 14 mg
- store between 20-25°C (68-77°F). Protect from light. Store in outer carton until contents used.
-
do not use if bottle wrap, or foil inner seal imprinted “Safety Seal®” is broken or missing
- see bottom panel for lot number and expiration date
Inactive ingredients
anhydrous citric acid, D&C red #33, FD&C red #40, flavors, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucrose
Questions or comments?
call 1-888-474-3099
PediaCare Children’s Allergy Bubblegum Flavor
Drug Facts
Active ingredient
(in each 5 mL, 1 teaspoon)
Diphenhydramine HCI 12.5 mg
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use
if the child has
- glaucoma
- a breathing problem such as chronic bronchitis
Ask a doctor or pharmacist before use
if the child is taking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- sedatives and tranquilizers may increase drowsiness
- excitability may occur, especially in children
Keep out of reach of children
In case of overdose, seek professional assistance or contact a Poison Control Center (1-800-222-1222) immediately.
Directions
- use only enclosed dosing cup designed for use with this product. Do not use any other dosing device.
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
- tsp = teaspoon, mL = milliliter, mg = milligram
| Age (yr) | Dose (tsp) |
| children 6 to 11 years | 1 to 2 teaspoonfuls (5 mL to 10 mL) |
| children 4 to 5 years | Do not use unless directed by a doctor |
| children under 4 years | do not use |
Other information
-
each teaspoon contains: sodium 6 mg
- store at room temperature
Inactive ingredients
citric acid, flavors, glycerin, poloxamer 407, purified water, red 33, red 40, sodium benzoate, sodium chloride, sodium citrate, sugar
Questions or comments?
call 1-888-474-3099
Principal Display Panel
See New Warnings & Directions
PediaCare®Children’s Allergy
Diphenhydramine HCI oral solution
Antihistamine/Cough suppressant
Cherry Flavor Liquid 4 FL OZ (118 mL)
Principal Display Panel
NO ACETAMINOPHEN
PediaCare®Children’s Allergy
Diphenhydramine HCI Oral Solution Antihistamine
BubbleGum Flavor 4 FL OZ (118 mL)
Prestige Brands Holdings, Inc.