Label: CLEARSKIN PROFESSIONAL DAILY CORRECTING- salicylic acid lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 10096-0154-1, 10096-0154-2, 10096-0154-3, 10096-0154-4 - Packager: New Avon LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
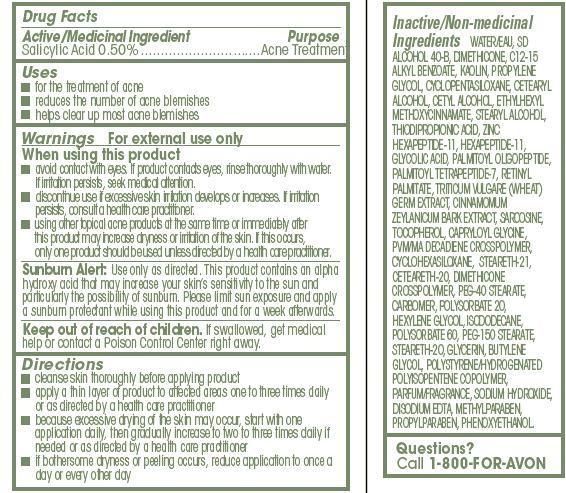
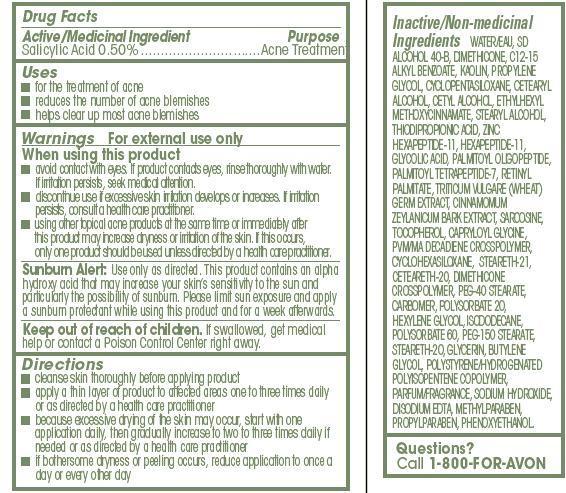
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using this product
- avoid contact with eyes. If product contacts eyes, rinse thoroughly with water.If irritation persists, seek medical attention.
- discontinue use if excessive skin irritation develops or increases. If irritationpersists, consult a health care practitioner.
- using other topical acne products at the same time or immediately afterthis product may increase dryness or irritation of the skin. If this occurs,only one product should be used unless directed by a health care practitioner
-
DOSAGE & ADMINISTRATION
Directions
- cleanse skin thoroughly before applying product
- apply a thin layer of product to affected areas one to three times daily or as directed by a health care practitioner
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a health care practitioner
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
INACTIVE INGREDIENT
Inactive/Non-medicinal
Ingredients WATER/EAU, SD ALCOHOL 40-B, DIMETHICONE, C12-15 ALKYL BENZOATE, KAOLIN, PROPYLENE GLYCOL, CYCLOPENTASILOXANE, CETEARYL ALCOHOL, CETYL ALCOHOL, ETHYLHEXYL
METHOXYCINNAMATE, STEARYL ALCOHOL, THIODIPROPIONIC ACID, ZINC HEXAPEPTIDE-11, HEXAPEPTIDE-11, GLYCOLIC ACID, PALMITOYL OLIGOPEPTIDE, PALMITOYL TETRAPEPTIDE-7, RETINYL PALMITATE, TRITICUM VULGARE (WHEAT) GERM EXTRACT, CINNAMOMUM ZEYLANICUM BARK EXTRACT, SARCOSINE, TOCOPHEROL, CAPRYLOYL GLYCINE, PVM/MA DECADIENE CROSSPOLYMER, CYCLOHEXASILOXANE, STEARETH-21, CETEARETH-20, DIMETHICONE CROSSPOLYMER, PEG-40 STEARATE, CARBOMER, POLYSORBATE 20, HEXYLENE GLYCOL, ISODODECANE, POLYSORBATE 60, PEG-150 STEARATE, STEARETH-20, GLYCERIN, BUTYLENE GLYCOL, POLYSTYRENE/HYDROGENATED POLYISOPENTENE COPOLYMER, PARFUM/FRAGRANCE, SODIUM HYDROXIDE, DISODIUM EDTA, METHYLPARABEN, PROPYLPARABEN, PHENOXYETHANOL. - QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLEARSKIN PROFESSIONAL DAILY CORRECTING
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10096-0154 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10096-0154-2 1 in 1 CARTON 11/09/2011 1 NDC:10096-0154-1 60 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:10096-0154-4 1 in 1 CARTON 11/09/2011 2 NDC:10096-0154-3 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 11/09/2011 Labeler - New Avon LLC (080143520) Establishment Name Address ID/FEI Business Operations Fareva Morton Grove, Inc. 116752326 manufacture(10096-0154)