INFANT GAS RELIEF DYE FREE- simethicone suspension
AmerisourceBergen (Good Neighbor Pharmacy) 46122
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
relieves the discomfort of infant gas frequently caused by air swallowing or certain formulas or foods
Warnings
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddenings
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains acetamnophen. Severe liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Directions
- shake well before using
- mL=milliliter
- measure only with enclosed syringe provided. Do not use any other dosing device
- keep dosing device with product
- replace cap tightly to maintain child resistance
- remove cap and insert syringe into hole at top of the bottle and turn upside down
- pull back syringe until filled to the prescribed level. If you pass the prescribed level, simply push syringe back until have reached the desired level. Slowly dispense the liquid into your child's mouth (towards inner cheek)
- dosage can also be mixed with 1 oz, cool water, infant formula, or other suitable liquids
- clean syringe well after use
- all dosages may be repeated as needed, after meals and at bedtime, or as directed by a physician. Do not exceed 12 doses per day
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age
| Weight (lb) | Age (yr) | Dose (mL)* |
| under 24 | under 2 years | 0.3 mL |
| 24 | over 2 years |
0.6 mL |
Inactive ingredients
citric acid, carboxymethylcellulose sodium, flavors, maltitol, microcrystalline cellulose, purified water, sodium benzoate, sodium citrate, xanthan gum
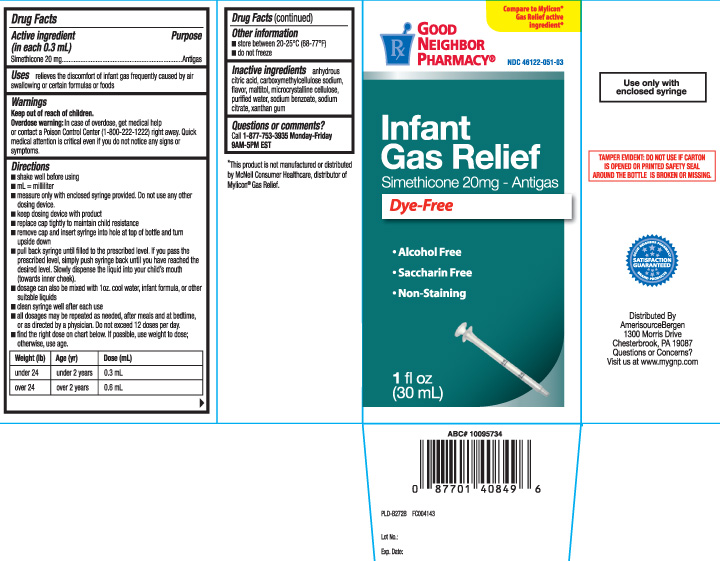
Principal Display Panel
Compare to Mylicon® Gas Relief active ingredient*
Infant Gas Relief
Simethicone 20mg - Antigas
Dye-Free
- Alcohol Free
- Saccharin Free
- Non-Staining
FL OZ (mL)
Use only with enclosed syringe
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND THE BOTTLE IS BROKEN OR MISSING
*This product is not manufactured or distributed by McNeil Consumer Healthcrae, distributor of Mylicon® Gas Relief.
Distributed by AmerisourceBergen
1300 Morris Drive
Chesterbrook, PA 19087
Visit us at www.mygnp.com
| INFANT GAS RELIEF
DYE FREE
simethicone suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - AmerisourceBergen (Good Neighbor Pharmacy) 46122 (007914906) |