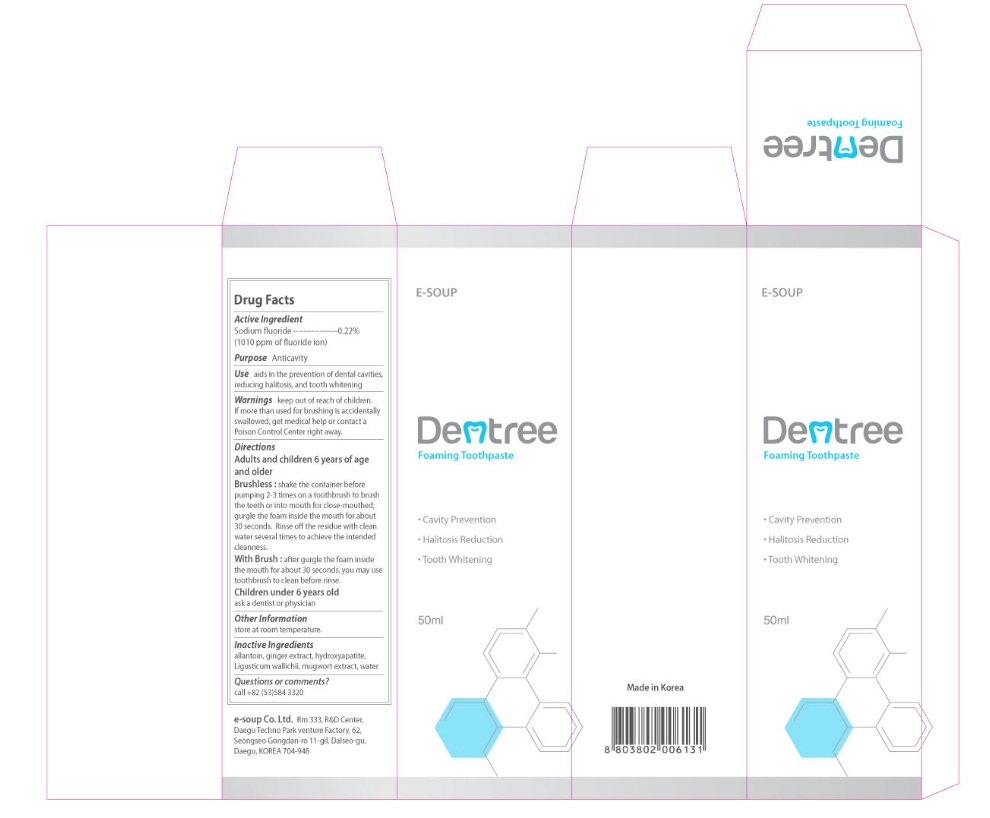
Label: DENTREE- sodium fluoride gel, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 76170-101-50 - Packager: E-SOUP Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
-
Directions
Adults and children 6 years of age and older
Brushless: shake the container before pumping 2-3 times on a toothbrush to brush the teeth or into mouth for close-mouthed, gurgle the foam inside the mouth for about 30 seconds. Rinse off the residue with clean water several times to achieve the intended cleanliness.
With Brush: after gurgle the foam inside the mouth for about 30 seconds, you may use toothbrush to clean before rinse.
Children under 6 years old
ask a dentist or physician - Other Information
- Inactive Ingredients
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DENTREE
sodium fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76170-101 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) GINGER (UNII: C5529G5JPQ) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) LIGUSTICUM WALLICHII WHOLE (UNII: 8Y1N3NX2DW) ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) Product Characteristics Color blue Score Shape Size Flavor VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76170-101-50 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 04/01/2012 Labeler - E-SOUP Co., Ltd. (557805059) Establishment Name Address ID/FEI Business Operations E-SOUP Co Ltd 557805059 manufacture(76170-101)