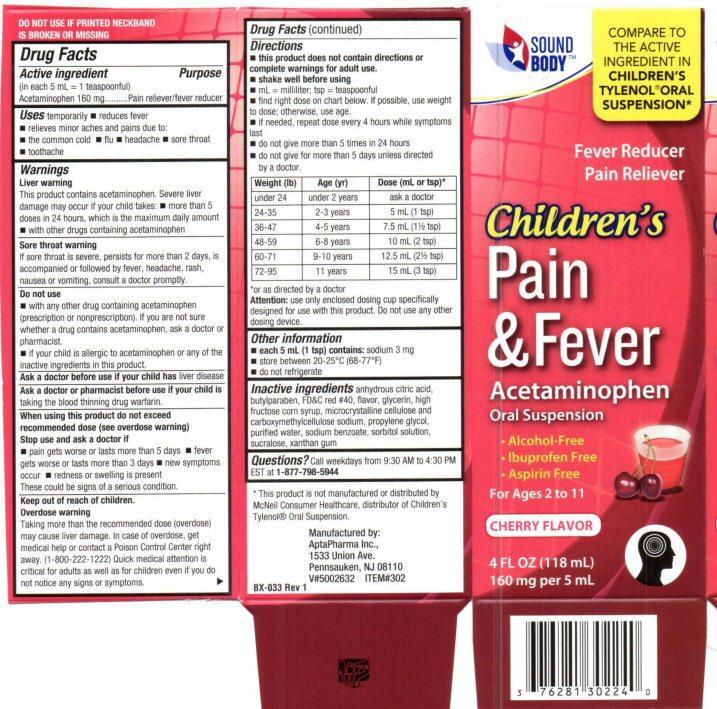
Label: CHILDRENS PAIN AND FEVER- acetaminophen liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 76281-302-24 - Packager: AptaPharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 28, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
Liver warning
This product contains acetaminophen.Severe liver damage may occur if your child takes:
more than 5 doses in 24 hours, which is the maximum daily amount
with other drugs containing acetaminophenSore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
- Do not use
- Ask a doctor before use if your child has
- Ask a doctor or pharmacist before use if your child is
- When using this product
- Stop use and ask a doctor if
- Overdose warning
-
Directions
this product does not contain directions or complete warnings for adult use
shake well before using
mL = milliliter; tsp = teaspoonful
find the right dose on the chart below. If possible, use weight to dose, otherwise, use age.
if needed, repeat dose every 4 hours while symptoms last
do not give more than 5 times in 24 hours
do not give for more than 5 days unless directed by a doctor.Weight (lb) Age (yr) Dose (mL or tsp)*
under 24 under 2 years ask a doctor
24-35 2 - 3 years 5 mL (1 tsp)
36-47 4 - 5 years 7.5 mL (1 1/2 tsp0
48-59 6 - 8 years 10 mL (2 tsp)
60-71 9 - 10 years 12.5. mL (2 1/2 TSP)
72-95 11 years 15 mL (3 tsp)
*or as directed by a doctor
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
- Other information
- Inactive ingredients
- Questions?
-
Product Label
SOUND BODY™
COMPARE TO THE ACTIVE INGREDIENTS IN CHILDREN'S TYLENOL® ORAL SUSPENSION
Children's
Pain and Fever
Acetaminophen
Oral Suspension
Alcohol Free
Ibuprofen Free
Aspirin FreeFor Ages 2 to 11
CHERRY FLAVORED
4 FL OZ (118 mL)
160 mg per 5 mLDO NOT USE IF PRINTED NECKBAND IA BROKEN OR MISSING
*This product is not manufactures or distributed by McNeil Consumer Healthcare, distributor of Children's Tylenol® Oral Suspension.
Manufactured by:
AptaPharma Inc.,
1533 Union Ave
Pennsauken, NJ 08110V#5002632 ITEM#302
BX-003 Rev 1

res
-
INGREDIENTS AND APPEARANCE
CHILDRENS PAIN AND FEVER
acetaminophen liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76281-302 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLPARABEN (UNII: 3QPI1U3FV8) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76281-302-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/21/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 08/21/2013 Labeler - AptaPharma Inc. (790523323) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(76281-302)