SOFT CARE NEUTRA-GERM ANTIBACTERIAL FRAGRANCE FREE- triclosan liquid
Diversey, Inc.
----------
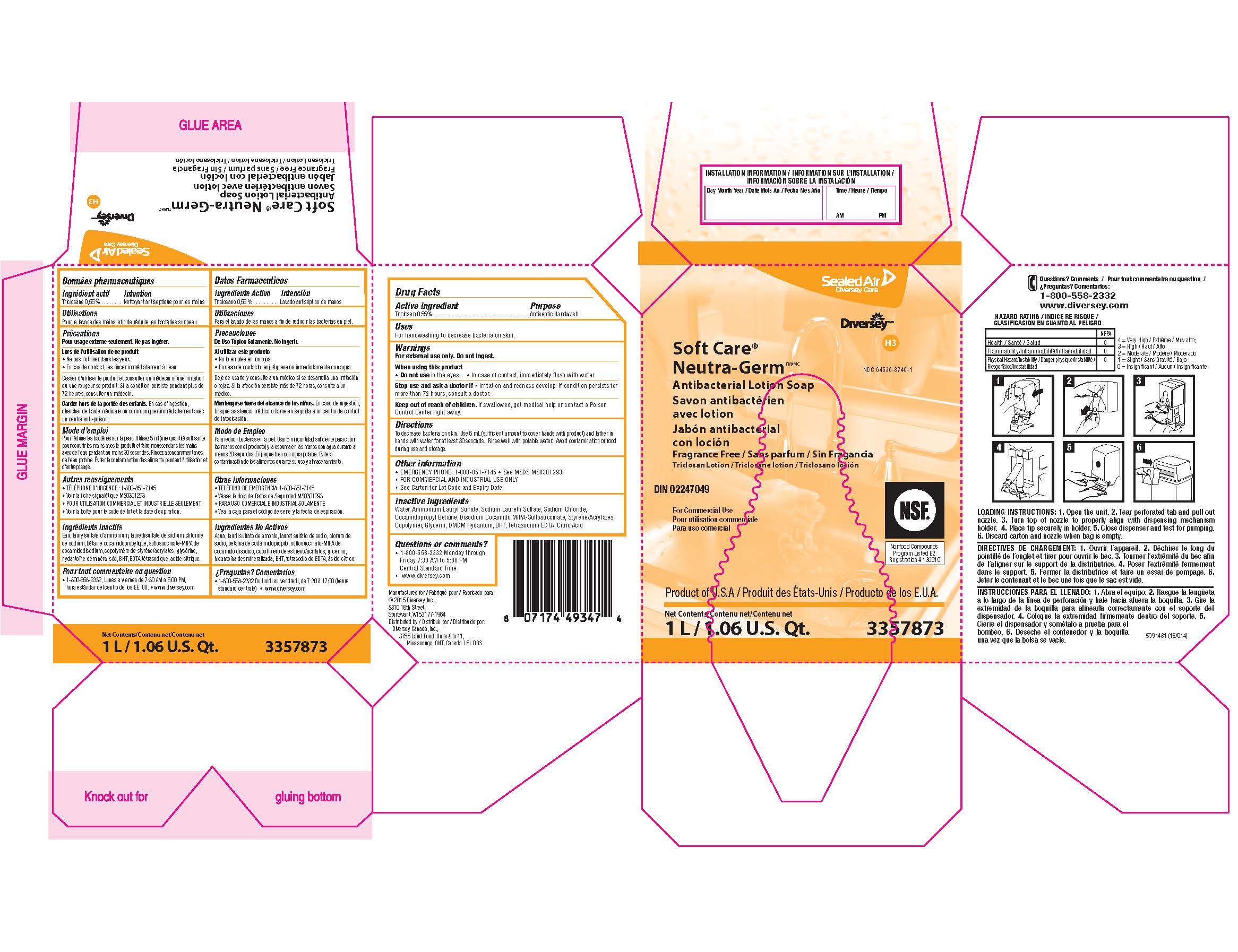
Drug Facts
Warnings
For external use only. Do not ingest.
Directions
To decrease bacteria on skin.
Use 5 mL (sufficient amount to cover hands with product) and lather in hands with water for at least 30 seconds.
Rinse well with potable water.
Avoid contamination of food during use and storage.
Other information
EMERGENCY PHONE: 1-800-851-7145
See MSDS MS0301293
FOR COMMERCIAL AND INDUSTRIAL USE ONLY
See Carton for Lot Code and Expiry Date.
Inactive ingredients
Water, Ammonium Lauryl Sulfate, Sodium Laureth Sulfate, Sodium Chloride, Cocamidopropyl Betaine, Disodium Cocamido MIPA-Sulfosuccinate, Styrene/Acrylates Copolymer, Glycerin, DMDM Hydantoin, BHT, Tetrasodium EDTA, Citric Acid
| SOFT CARE NEUTRA-GERM ANTIBACTERIAL FRAGRANCE FREE
triclosan liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Diversey, Inc. (018240817) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kutol Products Company Inc | 004236139 | manufacture(64536-8740) | |