BACTOSHIELD- chlorhexidine gluconate sponge
STERIS Corporation
----------
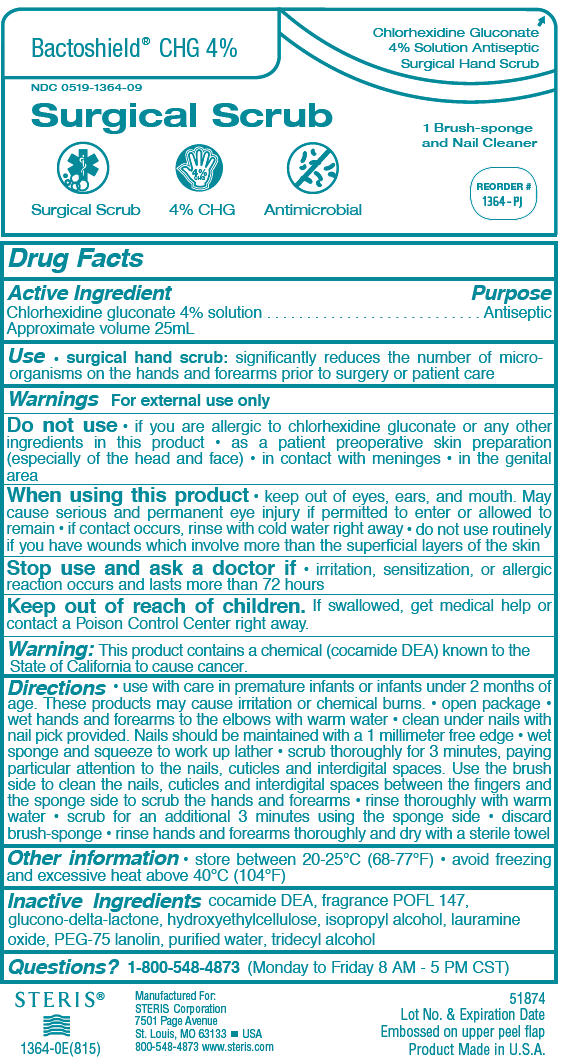
Bactoshield® CHG 4% Surgical Scrub
Uses
- surgical hand scrub: significantly reduces the number of micro-organisms on the hands and forearms prior to surgery or patient care
Warnings
For external use only
Do not use
- if you are allergic to chlorhexidine gluconate or any other ingredients in this product
- as a patient preoperative skin preparation (especially of the head and face)
- in contact with meninges
- in the genital area
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter or allowed to remain
- if contact occurs, rinse with cold water right away
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
Stop use and ask a doctor if
- irritation, sensitization, or allergic reaction occurs and lasts more than 72 hours
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- open package
- wet hands and forearms to the elbows with warm water
- clean under nails with nail pick provided. Nails should be maintained with a 1 millimeter free edge
- wet sponge and squeeze to work up lather
- scrub thoroughly for 3 minutes, paying particular attention to the nails, cuticles and interdigital spaces. Use the brush side to clean the nails, cuticles and interdigital spaces between the fingers and the sponge side to scrub the hands and forearms
- rinse thoroughly with warm water
- scrub for an additional 3 minutes using the sponge side
- discard brush-sponge
- rinse hands and forearms thoroughly and dry with a sterile towel
Other information
- store between 20-25°C (68-77°F)
- avoid freezing and excessive heat above 40°C (104°F)
Inactive Ingredients
cocamide DEA, fragrance POFL 147, glucono-delta-lactone, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, PEG-75 lanolin, purified water, tridecyl alcohol
PRINCIPAL DISPLAY PANEL - 25 mL Applicator Package Label
Bactoshield ® CHG 4%
Chlorhexidine Gluconate
4% Solution Antiseptic
Surgical Hand Scrub
NDC 0519-1364-09
Surgical Scrub
1 Brush-sponge
and Nail Cleaner
REORDER #
1364 - PJ
Surgical Scrub
4% CHG
Antimicrobial
STERIS®
1364-0E(815)
Manufactured For:
STERIS Corporation
7501 Page Avenue
St. Louis, MO 63133 ▪ USA
800-548-4873 www.steris.com
51874
Lot No. & Expiration Date
Embossed on upper peel flap
Product Made in U.S.A.
| BACTOSHIELD
chlorhexidine gluconate sponge |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - STERIS Corporation (139424188) |
Revised: 1/2019
Document Id: c481a645-4967-4efb-a94a-7e7a2079487f
Set id: ec19f244-662c-4643-8be0-6d96a0d07801
Version: 4
Effective Time: 20190128
STERIS Corporation