Label: COLD SORE TREATMENT- benzalkonium chloride tincture
-
Contains inactivated NDC Code(s)
NDC Code(s): 37205-973-19 - Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
-
WARNINGS
For external use only. Flammable, keep away from fire or flame.
Do not use
- in the eyes
- over large areas of the body
- if you are allergic to any ingredient in the product
- more than 3 times per day
- longer than one week unless directed by a doctor.
Ask a doctor if
- used to treat deep or puncture wounds, animal bites, or serious burns
- you are pregnant or nursing a baby
-
DIRECTIONS
Adults and children over 2 years of age:
- Clean the lip area of any lip preparations, lotions, ointments, residual beverages, or cosmetics including lipstick using warm water and a washcloth
- Remove the cardboard top from applicator and place onto the glass/plastic vial end - opposite the brush end of the product
- Squeeze the cardboard to break the inner glass vial open
- Saturate the applicator end with solution by holding the brush end down and squeezing the container until you can see liquid on the brush applicator
- For best results, massage the solution into the cold sore by rubbing. Rub firmly, but take care not to damage the tissue. The purpose of the rubbing is to deliver the drug to the infection site. Hold the vial so the solution flows to the sore.
- To treat most cold sores, usually one treatment is enough.
- If your symptoms go away and then return later, apply another dose.
- Do not use more than 3 times per day
- Discard after use.
- SPL UNCLASSIFIED SECTION
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS
-
PACKAGE INFORMATION - TRIFOLD INNER
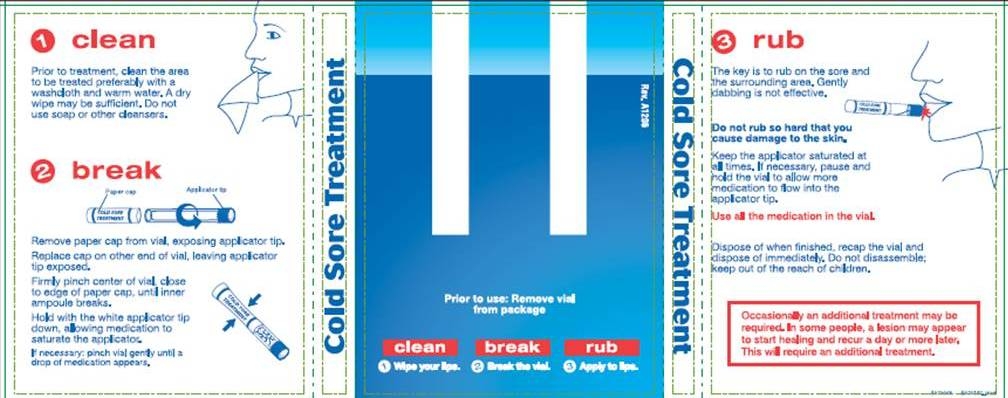
1. CLEAN
Prior to treatment, clean the area to be treated preferably with a washcloth and warm water. A dry wipe may be sufficient. Do not use soap or other cleansers.
2. BREAK
Remove paper cap from vial, exposing applicator tip.
Replace cap on other end of vial, leaving applicator tip exposed.
Firmly pinch center of vial, close to edge of paper cap, until inner ampoule breaks.
Hold with the white applicator tip down, allowing medication to saturate the applicator.
If necessary: pinch vial gently until a drop of medication appears.
Prior to use: Remove vial from package
3. RUB
The key is to rub on the sore and the surrounding area. Gently dabbing is not effective.
Do not rub so hard that you cause damage to the skin.
Keep the applicator saturated at all times. If necessary, pause and hold the vial to allow more medication to flow into the applicator tip.
Use all the medication in the vial.
Dispose of when finished, recap the vial and dispose of immediately. Do not disassemble; keep out of reach of children.
Occasionally an additional treatment may be required. In some people, a lesion may appear to start healing and recur a day or more later. This will require an additional treatment.
-
PACKAGE INFORMATION - TRIFOLD OUTER
LEADER SATISFACTION GUARANTEED
NDC 37205-973-19
Cold Sore
Treatment
Cold Sore / Fever Blister Treatment
Topical Antiseptic
Treats most cold sores in just ONE treatment
Unique patented applicator eliminates need to touch sore with your fingers
No messy creams or ointments left on the face
Single use applicator
Compare to Viroxyn©2 VIALS (0.6 ml EACH)
Fast Pain Relief
Distributed by Cardinal Health
Dublin, OH 43017 CIN 3924091
www.myleader.com MADE IN USA
All Leader Brand products are 100% satisfaction guaranteed or return to place of purchase for a full refund.
US Patent No. 6,211,243B1
A patented new approach (US 6,211,243B1) to treating cold sores - rubbing the cold sore with the applicator tip delivers the germicidal medicine directly to the site of the infection.
Cold Sores and Fever Blisters are both caused by the Herpes Simplex virus (typically HSV-1) and are just different popular names for the HSV-caused lesions.
HERPES IS HIGHLY CONTAGIOUS.
A well-intentioned kiss can transfer the virus to another individual. Children are highly at risk.
After touching a cold sore:
* Wash your hands immediately with warm water and soap
* Do not touch your eyes
* Do not touch your genitals or other parts of the body. It is possible to transfer the virus to other parts of the body (autoinoculation)
Occasionally an additional treatment may be required. In some people, a lesion may appear to start healing and recur a day or more later. This will require an additional treatment.
-
INGREDIENTS AND APPEARANCE
COLD SORE TREATMENT
benzalkonium chloride tinctureProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37205-973 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzalkonium chloride (UNII: F5UM2KM3W7) (Benzalkonium - UNII:7N6JUD5X6Y) Benzalkonium chloride 0.13 mL in 1 mL Inactive Ingredients Ingredient Name Strength isopropyl alcohol (UNII: ND2M416302) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37205-973-19 2 in 1 PACKAGE 1 0.6 mL in 1 VIAL, PATENT DELIVERY SYSTEM Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/20/2007 Labeler - Cardinal Health (097537435)


