MEDI-FIRST BURN FIRST AID- benzethonium chloride, benzocaine, and menthol aerosol, spray
Unifirst First Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
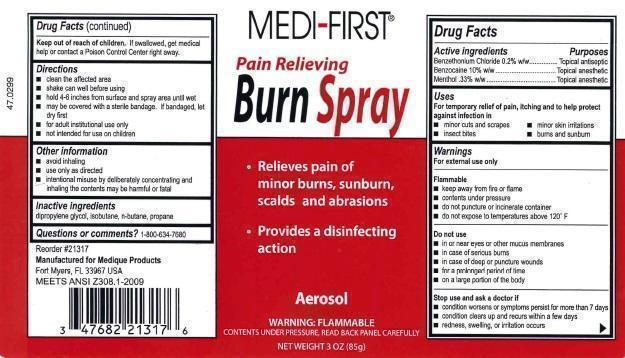
Medi-First Burn Spray
Uses
For temporary relief of pain, itching and to help protect against infection in
minor cuts and scrapes
minor skin irritations
insect bites
burns and sunburn
Warnings
For external use only
Flammable
keep away from fire or flame
contents under pressure
do not puncture or incinerate container
do not expose to temperatures above 120° F
Do not use
in or near eyes or other mucus membranes
in case of serious burns
in case of deep or puncture wounds
for prolonged period of time
on a large portion of the body
Stop use and ask a doctor if
condition worsens or symptoms persist for more than 7 days
condition clears up and recurs within a few days
redness, swelling, or irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
clean the affected area
shake can well before using
hold 4-6 inches from surface and spray area until wet
may be covered with a sterile bandage. If bandaged, let dry first
for adult institutional use only
not intended for use on children
| MEDI-FIRST BURN FIRST AID
benzethonium chloride, benzocaine, and menthol aerosol, spray |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Unifirst First Aid Corporation (832947092) |
| Registrant - Dixon Investments, Inc. (115315822) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dixon Investments, Inc. | 115315822 | manufacture(47682-213) | |