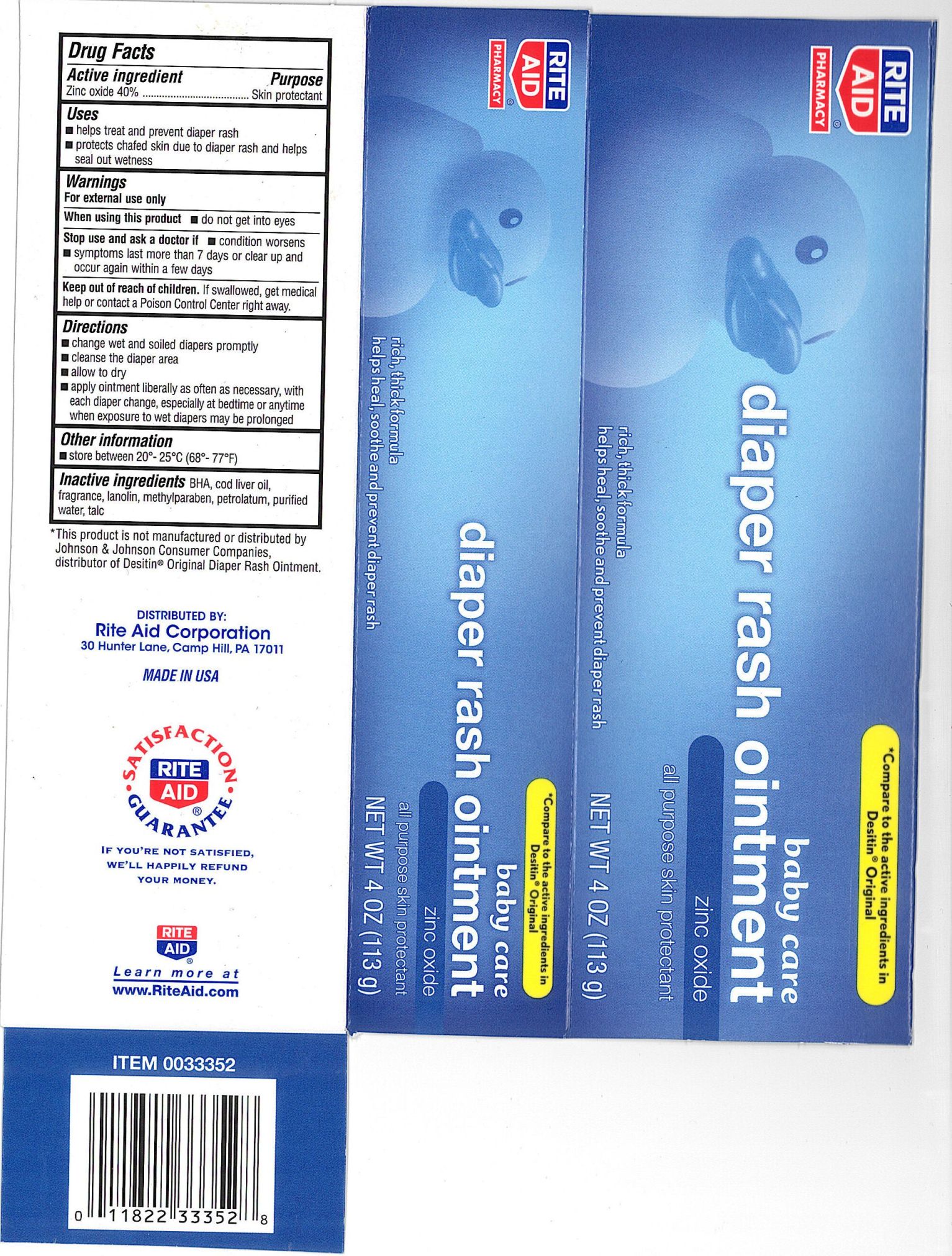
Label: RITE AID DIAPER RASH- zinc oxide ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 11822-0376-3 - Packager: RiteAid Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 20, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
When using this product - do not get into eyes
Stop use and ask a doctor if:
- condition worsens - symptoms last more than 7 days or clear
up and occur again within a few days.
Keep out of the reach of children. If swallowed get medical help or contact a Poison Control Center right away
-
DOSAGE & ADMINISTRATION
Directions
- change wet and soiled diapers promptly
- cleanse the diaper area
- allow to dry
- apply ointment liberally as often as necessary, with each diaper change,
especially at bedtime or anytime when exposure to wet diapers may be prolonged
Other information
Store at 20 degrees to 25 degrees C (68 degrees to 77 degrees F)
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RITE AID DIAPER RASH
zinc oxide ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-0376 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 400 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) METHYLPARABEN (UNII: A2I8C7HI9T) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) WATER (UNII: 059QF0KO0R) TALC (UNII: 7SEV7J4R1U) COD LIVER OIL (UNII: BBL281NWFG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-0376-3 1 in 1 CARTON 1 113 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 07/20/2010 Labeler - RiteAid Corporation (014578892) Registrant - Pharma Pac, LLC (140807475) Establishment Name Address ID/FEI Business Operations Pharma Pac, LLC 140807475 manufacture