ACETAMINOPHEN- acetaminophen tablet
Gemini Pharmaceuticals, Inc. dba ONDRA Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
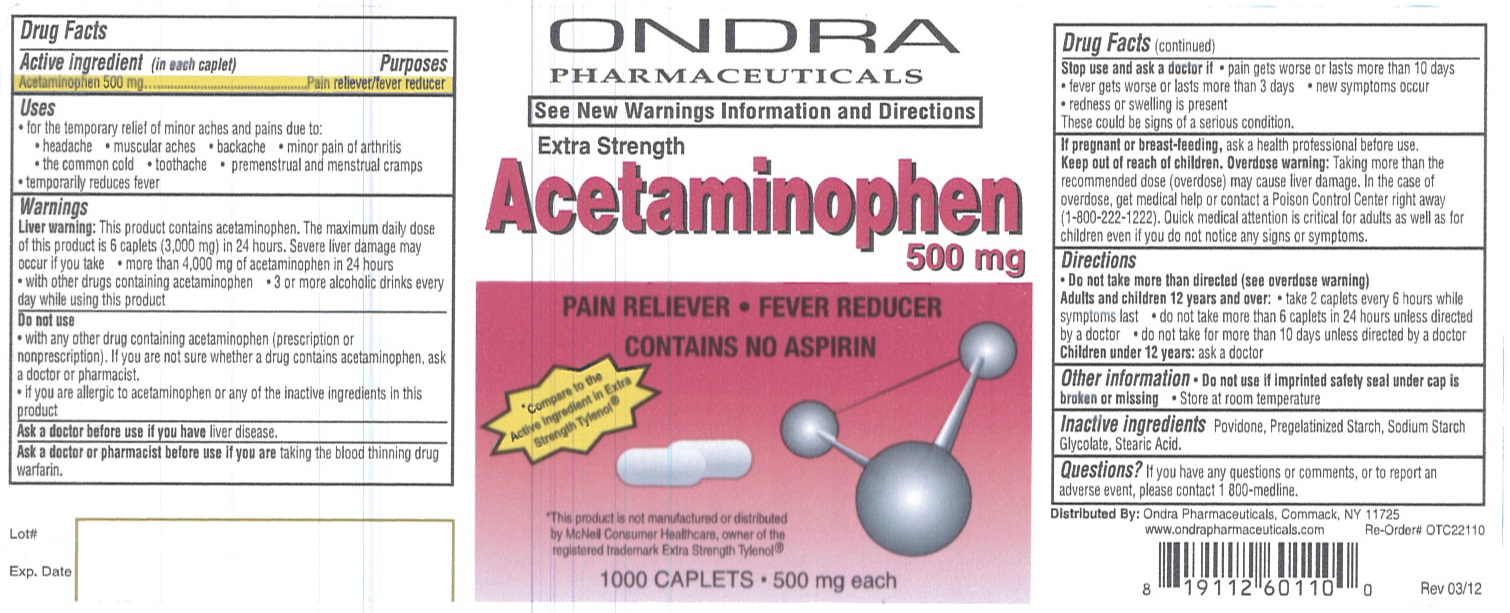
Ondra Acetaminophen 500 mg Caplets
Uses
- for the temporary relief of minor aches and pains due to:
- headache
- muscular aches
- backache
- minor pain of arthritis
- the common cold
- toothache
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 6 caplets (3,000 mg) in 24 hours. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or non prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In the case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
-
Do not take more than directed (see overdose warning)
Adults and children 12 years and over: - take 2 caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours unless directed by a doctor
- do not take for more than 10 days unless directed by a doctor
Children under 12 years: ask a doctor
Other information
-
Do not use if imprinted safety seal under cap is broken or missing.
- Store at room temperature
Questions?
If you have any questions or comments, or to report an adverse event, please contact 1 800-medline.
Principal Display Panel
ONDRA PHARMACEUTICALS
See New Warnings Information and Directions
Extra Strength
Acetaminophen 500 mg
PAIN RELIEVER
FEVER REDUCER
CONTAINS NO ASPIRIN
Compare to the Active ingredient in Extra Strength Tylenol®
This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol®
1000 CAPLETS 500 mg each
| ACETAMINOPHEN
acetaminophen tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Gemini Pharmaceuticals, Inc. dba ONDRA Pharmaceuticals (055942270) |
| Registrant - Gemini Pharmaceuticals, Inc. dba ONDRA Pharmaceuticals (055942270) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gemini Pharmaceuticals, Inc. dba ONDRA Pharmaceuticals | 055942270 | manufacture(51645-601) | |