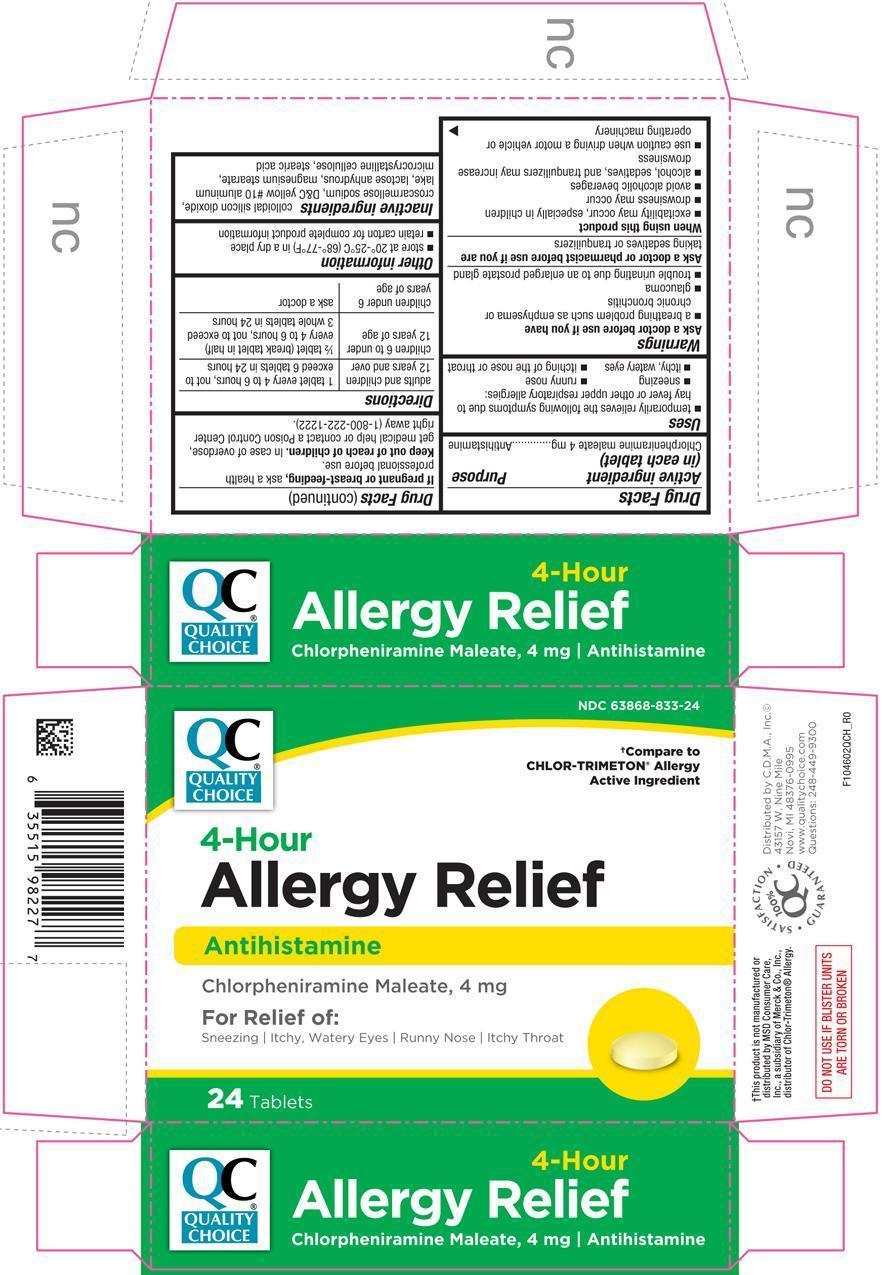
ALLERGY RELIEF- chlorpheniramine maleate tablet, coated
Chain Drug Marketing Association
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
QCH - 1046 - 2019-1004
Uses
- temporarily relieves the following symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- runny nose
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
| adults and children 12 years and over | 1 tablet every 4 to 6 hours, not to exceed 6 tablets in 24 hours |
| children 6 to under 12 years of age | ½ tablet (break tablet in half) every 4 to 6 hours, not to exceed 3 whole tablets in 24 hours |
| children under 6 years of age | ask a doctor |
Other information
- store at 20°-25°C (68°-77°F) in a dry place
- retain carton for complete product information
| ALLERGY RELIEF
chlorpheniramine maleate tablet, coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Chain Drug Marketing Association (011920774) |
Revised: 10/2021
Document Id: cdc44784-5b90-d65c-e053-2995a90ad6e8
Set id: e8d2ec82-806e-4755-b8af-40380ce33271
Version: 3
Effective Time: 20211007
Chain Drug Marketing Association