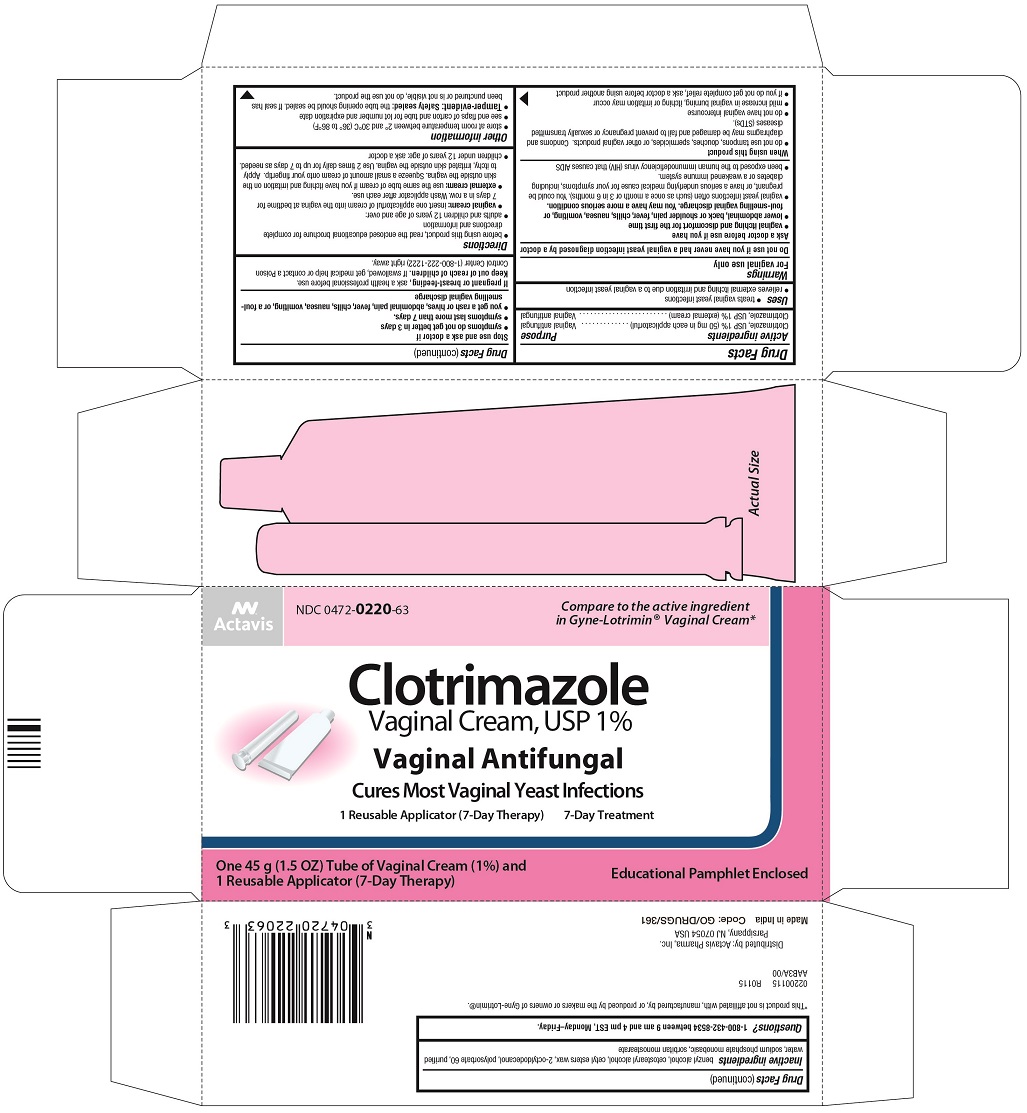
CLOTRIMAZOLE- clotrimazole cream
Actavis Pharma, Inc.
----------
Clotrimazole Vaginal Cream, USP
Uses
- treats vaginal yeast infections
- relieves external itching and irritation due to a vaginal yeast infection
Warnings
For vaginal use only
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant, or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
When using this product
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
- if you do not get complete relief, ask a doctor before using another product
Directions
- before using this product, read the enclosed educational brochure for complete directions and information
- adults and children 12 years of age and over:
- vaginal cream: insert one applicatorful of cream into the vagina at bedtime for 7 days in a row. Wash applicator after each use.
- external cream: use the same tube of cream if you have itching and irritation on the skin outside the vagina. Squeeze a small amount of cream onto your fingertip. Apply to itchy, irritated skin outside the vagina. Use 2 times daily for up to 7 days as needed.
- children under 12 years of age: ask a doctor
Other information
- store at room temperature between 2° and 30°C (36° to 86°F)
- see end flaps of carton and tube for lot number and expiration date
- Tamper-evident: Safety sealed: the tube opening should be sealed. If seal has been punctured or is not visible, do not use the product.
Inactive ingredients
benzyl alcohol, cetostearyl alcohol, cetyl esters wax, 2-octyldodecanol, polysorbate 60, purified water, sodium phosphate monobasic, sorbitan monostearate
Principal Display Panel
Actavis
NDC 0472-0220-63
Compare to the active ingredient
in Gyne-Lotrimin® Vaginal Cream*
Clotrimazole
Vaginal Cream, USP 1%
Vaginal Antifungal
Cures Most Vaginal Yeast Infections
1 Reusable Applicator (7-Day Therapy)
7-Day Treatment
One 45 g (1.5 OZ) Tube of Vaginal Cream (1%) and
1 Reusable Applicator (7-Day Therapy)
Educational Pamphlet Enclosed
| CLOTRIMAZOLE
clotrimazole cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Actavis Pharma, Inc. (119723554) |
Revised: 9/2022
Document Id: 2b9ed807-81d1-4e77-9b33-2ef870f0e73d
Set id: e8468943-1087-4317-8b64-236f15d7f465
Version: 10
Effective Time: 20220923
Actavis Pharma, Inc.