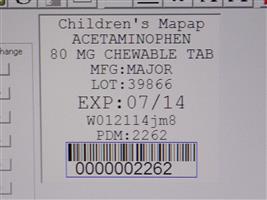
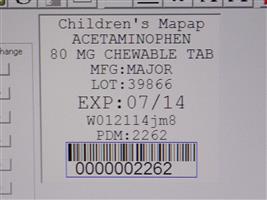
Label: CHILDRENS CHEWABLE MAPAP- acetaminophen tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 68151-2262-4 - Packager: Carilion Materials Management
- This is a repackaged label.
- Source NDC Code(s): 0904-5256
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 23, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each chewable tablet)
- Purposes
- Uses
-
Warnings
This product contains acetaminophen. Severe liver damage may occur if your child takes ■ more than 5 doses in 24 hours, which is the maximum daily amount ■ with other drugs containing acetaminophen. Liver warning:
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly. Sore throat warning:
■ with any other other drug containing acetaminophen (prescription or non prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist. ■ if your child is allergic to acetaminophen or any of the inactive ingredients in this product. Do not use
-
Directions
■ ■ Find right dose on the chart below. If possible, use weight to dose; otherwise, use age. ■ Chew before swallowing ■ If needed, repeat dose every 4 hours while symptoms lasts ■ Do not give more than 5 times in 24 hours ■ Do not give for more than 5 days unless directed by a doctor. This products does not contain directions or complete warnings for adult use.
Weight Age Tablets Under 24 lbs
Under 2 years
Ask a doctor
24-35 lbs
2-3 years
2 tablets
36-47 lbs
4-5 years
3 tablets
48-59 lbs
6-8 years
4 tablets
60-71 lbs
9-10 years
5 tablets
72-95 lbs
11 years
6 tablets
- Other Information
- Inactive Ingredients
- Mapap Acetaminophen 80mg chw TAB
-
INGREDIENTS AND APPEARANCE
CHILDRENS CHEWABLE MAPAP
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68151-2262(NDC:0904-5256) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 80 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) POVIDONES (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SORBITOL (UNII: 506T60A25R) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color pink Score no score Shape ROUND (ROUND BICONVEX TABLET) Size 10mm Flavor Imprint Code 1G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68151-2262-4 1 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 05/04/2011 Labeler - Carilion Materials Management (079239644) Registrant - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-2262)