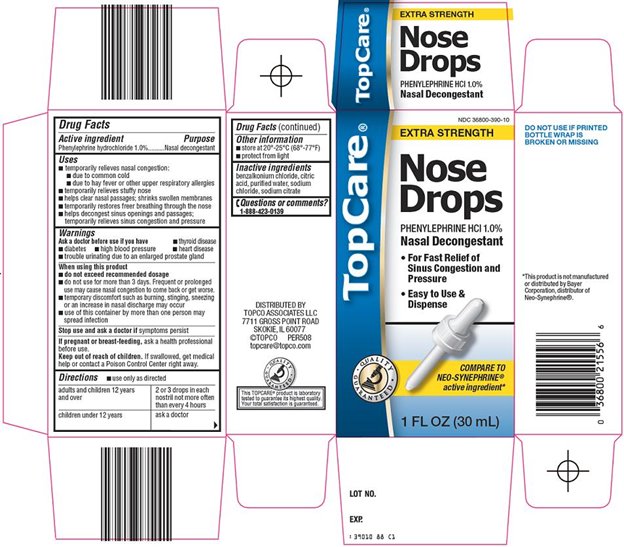
TOPCARE NOSE DROPS- phenylephrine hydrochloride liquid
Topco Associates LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Topco Nose Drops Drug Facts
Uses
- •
- temporarily relieves nasal congestion:
- •
- due to common cold
- •
- due to hay fever or other upper respiratory allergies
- •
- temporarily relieves stuffy nose
- •
- helps clear nasal passages; shrinks swollen membranes
- •
- temporarily restores freer breathing through the nose
- •
- helps decongest sinus openings and passages; temporarily relieves sinus congestion and pressure
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- diabetes
- •
- thyroid disease
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- do not exceed recommended dosage
- •
- do not use for more than 3 days. Frequent or prolonged use may cause nasal congestion to come back or get worse.
- •
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- •
- use of this container by more than one person may spread infection
Directions
- •
- use only as directed
|
adults and children 12 years and over |
2 or 3 drops in each nostril not more often than every 4 hours |
|
children under 12 years |
ask a doctor |
| TOPCARE NOSE DROPS
phenylephrine hydrochloride liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Topco Associates LLC (006935977) |
Revised: 11/2017
Document Id: 651298dd-f757-4585-ac46-027dd18d6bd8
Set id: e6ceb962-8355-4267-b133-2ffa77b16893
Version: 2
Effective Time: 20171110
Topco Associates LLC