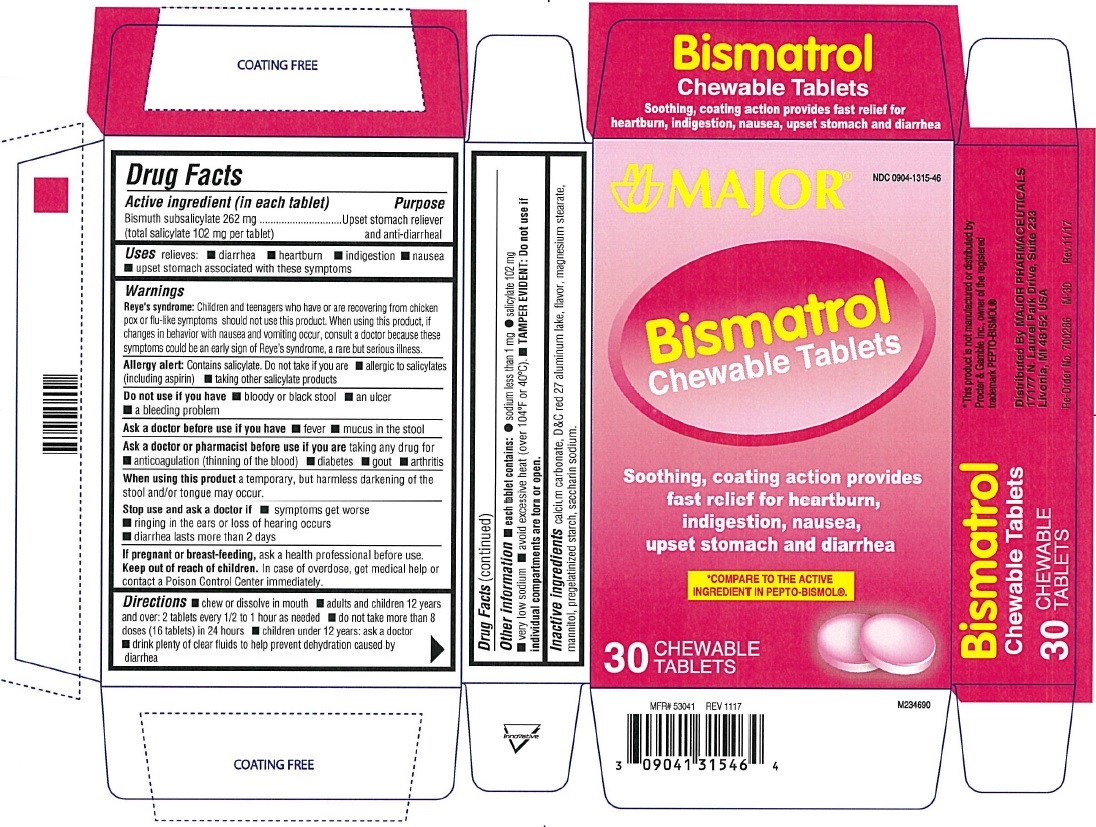
BISMATROL
- bismuth subsalicylate tablet, chewable
Major Pharmaceuticals
----------
Major Bismatrol Chewable Tablets
Active ingredient (in each tablet)
Bismuth subsalicylate 262 mg
(total salicylate 102 mg per tablet)
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. when using this product , if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
Ask a doctor or pharmacist before use if you are taking any drug for
- anticoagulation (thinning of the blood)
- diabetes
- gout
- arthritis
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center immediately.
Directions
- chew or dissolve in mouth
- adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed
- do not take more than 8 doses (16 tablets) in 24 hours
- children under 12 years: ask a doctor
- drink plenty of fluids to help prevent dehydration caused by diarrhea.
Other information:
each tablet contains:
- sodium less than 1 mg
- salicylate 102 mg
- very low sodium
- avoid excessive heat (over 104˚F or 40˚C)
- TAMPER EVIDENT:Do not use if individual compartments are torn or missing.
| BISMATROL
bismuth subsalicylate tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guardian Drug Company | 119210276 | MANUFACTURE(0904-1315) | |
Revised: 2/2024
Document Id: 6f4311ee-41fd-405e-bad3-ff802a4fc9e4
Set id: e5021687-11aa-472a-bd06-151415e1e2b3
Version: 7
Effective Time: 20240212
Major Pharmaceuticals