PAIN RELIEF EXTRA STRENGTH- acetaminophen tablet, film coated
Better Living Brands, LLC
----------
Signature Care 44-175-Delisted
Uses
- temporarily relieves minor aches and pains due to:
- headache
- the common cold
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Directions
- do not take more than directed
- adults and children 12 years and over
- take 2 caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours, unless directed by a doctor
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: ask a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
castor oil, hypromellose, povidone, sodium starch glycolate, starch, stearic acid
Principal Display Panel
Signature™
care
Quality Guaranteed
Compare to
Extra Strength
Tylenol® Caplets
active ingredient*
NDC 21130-975-29
Extra Strength
Pain Relief
ACETAMINOPHEN 500 mg
Pain Reliever /
Fever Reducer
• Contains no aspirin
Actual Size
150 CAPLETS
*This product is not manufactured or distributed by
Johnson & Johnson Corporation, distributors of
Extra Strength Tylenol® Caplets.
50844 REV0617A17529
DISTRIBUTED BY
BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
1-888-723-3929
www.betterlivingbrandsLLC.com
OUR PROMISE
QUALITY & SATISFACTION
100% GUARANTEED
OR YOUR MONEY BACK.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Signature Care 44-175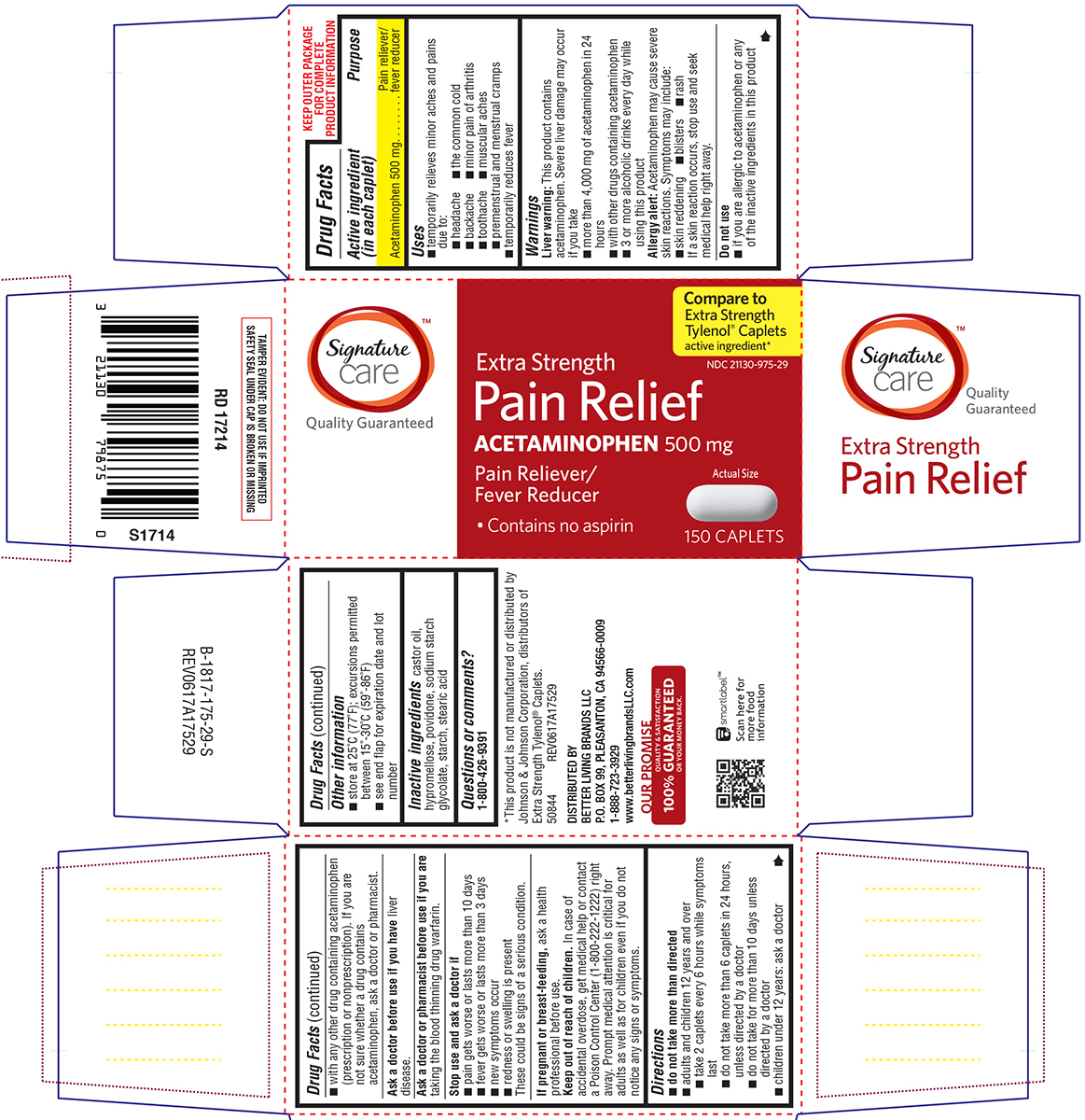
| PAIN RELIEF
EXTRA STRENGTH
acetaminophen tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Better Living Brands, LLC (009137209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(21130-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(21130-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(21130-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | manufacture(21130-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(21130-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117597853 | pack(21130-975) | |