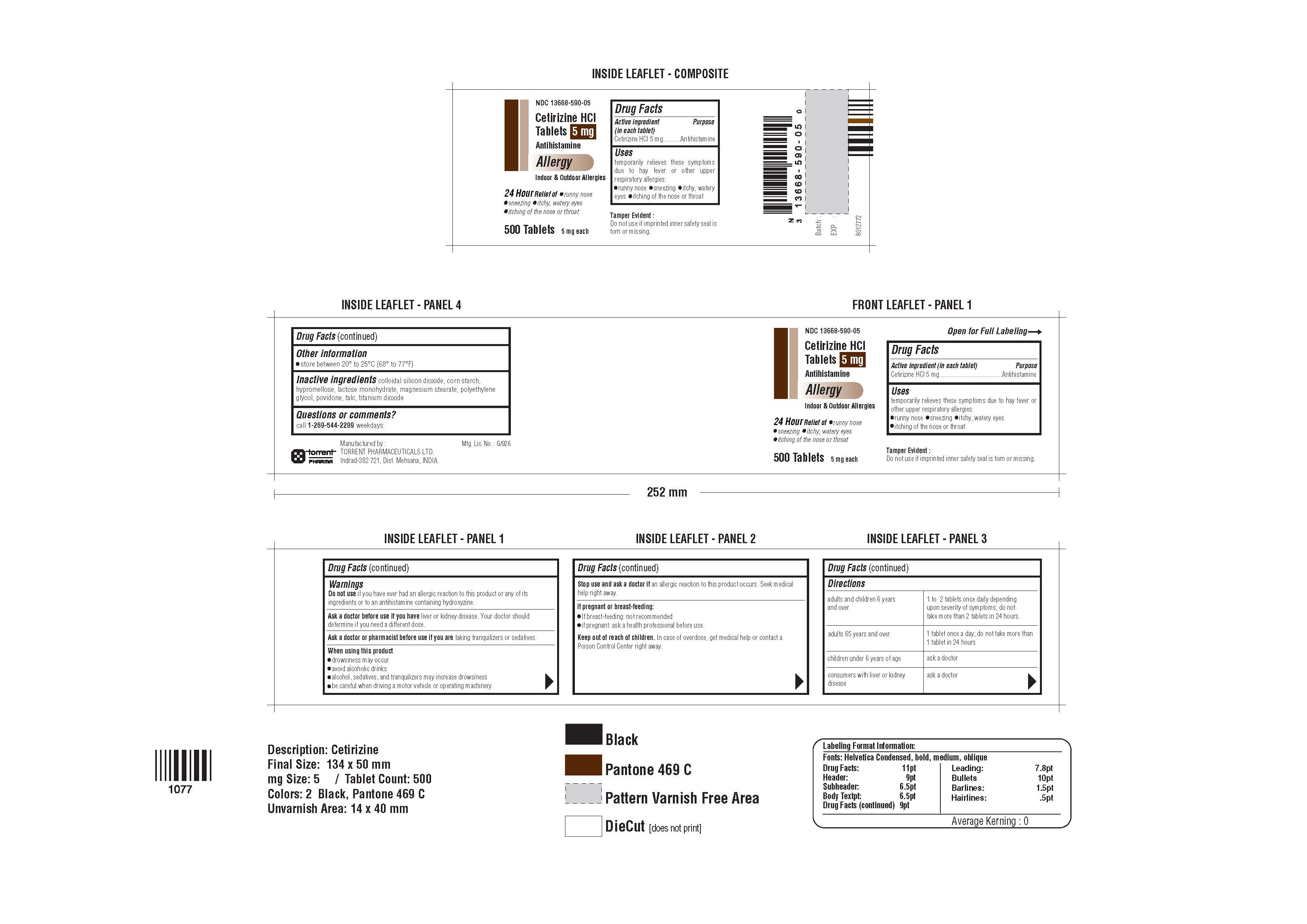
CETIRIZINE HYDROCHLORIDE- cetirizine hydrochloride tablet
Torrent Pharmaceuticals Limited
----------
Cetirizine Hydrochloride Tablets
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding:
- If breast-feeding: not recommended
- if pregnant: ask a health professional before use.
Directions
| adults and children 6 years and over
| 1 to 2 tablets once daily depending upon
severity of symptoms; do not take more than 2 tablets in 24 hours. |
| adults 65 years and over
| 1 tablet once a day; do not take more than
1 tablet in 24 hours |
| children under 6 years of age
| ask a doctor
|
| consumers with liver or kidney disease
| ask a doctor
|
- store between 20° to 25°C (68° to 77°F)
colloidal silicon dioxide, corn starch, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, povidone, talc, titanium dioxide
Questions or comments?
call 1-269-544-2299 weekdays
| CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Torrent Pharmaceuticals Limited (916488547) |
| Registrant - Torrent Pharma, Inc. (790033935) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Torrent Pharmaceuticals Limited | 916488547 | manufacture(13668-590) | |