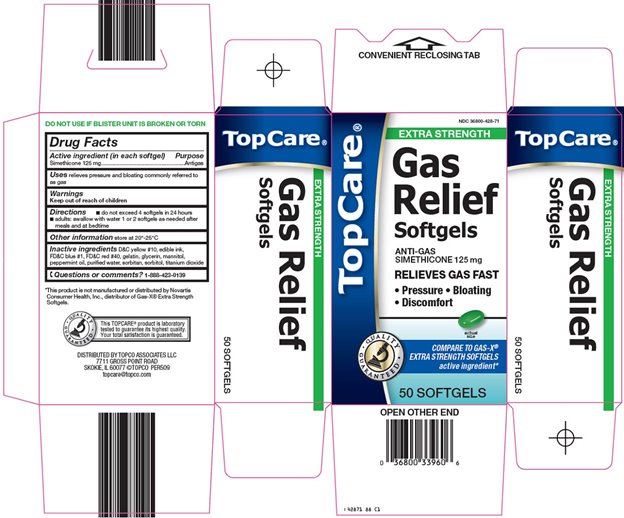
TOPCARE GAS RELIEF EXTRA STRENGTH- simethicone capsule, liquid filled
Topco Associates LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Topco Associates LLC. Gas Relief Drug Facts
Directions
- •
- do not exceed 4 softgels in 24 hours
- •
- adults: swallow with water 1 or 2 softgels as needed after meals and at bedtime
| TOPCARE GAS RELIEF
EXTRA STRENGTH
simethicone capsule, liquid filled |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Topco Associates LLC (006935977) |
Revised: 11/2017
Document Id: c9916875-40ad-4965-84f9-4f359ae4d6fe
Set id: e471a42d-3d56-41b1-a7ff-5609eae1559c
Version: 2
Effective Time: 20171110
Topco Associates LLC