STOOL SOFTENER- docusate sodium capsule, liquid filled
ARMY AND AIR FORCE EXCHANGE SERVICE
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Exchange Select 44-351 Delisted
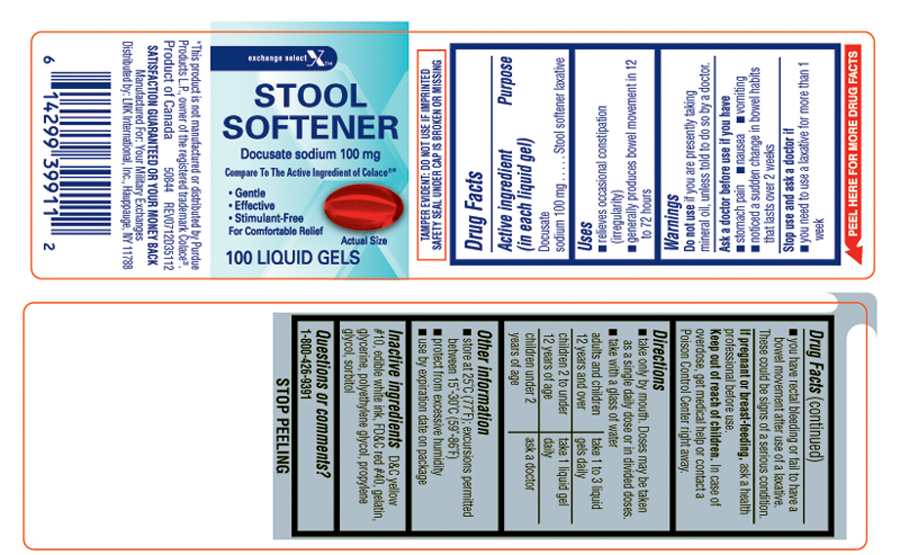
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
- take only by mouith. Doses may be taken as a single daily dose or in divided doses.
- take with a glass of water
| adults and children 12 years and over |
take 1 to 3 liquid gels |
| children 2 to under 12 years of age | take 1 liquid gel daily |
| children under 2 years of age | ask a doctor |
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from excessive humidity
- use by expiration date on package
Inactive ingredients
D&C yellow #10, edible white ink, FD&C red #40, gelatin, glycerine, polyethylene glycol, propylene glycol, sorbitol
Principal display panel
exchange select™
STOOL SOFTENER
Docusate sodium 100 mg
Compare To The Active Ingredient of Colace®*
• Gentle
• Effective
• Stimulant-Free
For Comfortable Relief
100 LIQUID GELS
*This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Colace®.
Product of Canada 50844 REV0712D35112
SATISFACTION GUARANTEED OR YOUR MONEY BACK
Manufactured For: Your Military Exchanges
Distributed by: LNK International, Inc., Hauppauge, NY 11788
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Exchange Select 44-351
| STOOL SOFTENER
docusate sodium capsule, liquid filled |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - ARMY AND AIR FORCE EXCHANGE SERVICE (001695568) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Accucaps Industries, Ltd. | 248441727 | MANUFACTURE(55301-351) | |