TANDEM - F
- ferrous fumarate and polysacchride iron complex and folic acid capsule
US Pharmaceutical Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
see all prescribing information for Tandem
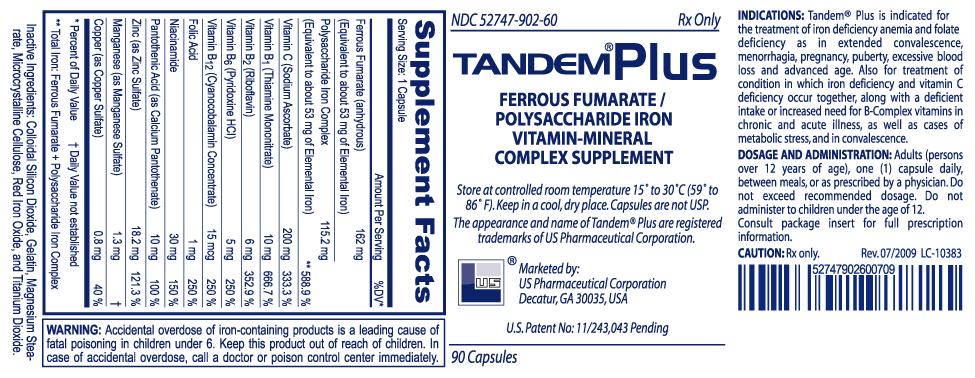
DESCRIPTION: Each capsule contains: Ferrous Fumarate (anhydrous) .191.1 mg Polysaccharide Iron Complex..135.9 mg (Equivalent to about 125 mg of elemental iron) Vitamin C (from ProAscorb C‡).40 mg Vitamin B3 (from ProAscorb C‡) ..3 mg Inactive Ingredients: Hypromellose, Magnesium Stearate, Titanium Dioxide,
CLINICAL PHARMACOLOGY: IntegraTM is unique in that it utilizes two (2) different forms of iron, i.e., Ferrous Fumarate and Polysaccharide Iron Complex (as cell-contracted akaganèite), making available a total of 125 mg of elemental iron per capsule as follows:
Ferrous Fumarate (anhydrous) 191.1 mg Polysaccharide iron complex (PIC) 135.9 mg
Ferrous Fumarate: Provides about 62.5 mg of elemental iron per dose. Ferrous Fumarate is an anhydrous salt of a combination of ferrous iron and fumaric acid, containing 33% of iron per weight. The acute toxicity in experimental animals is low and Ferrous Fumarate is well tolerated clinically. As a ferrous salt, it is more efficiently absorbed in the duodenum. Iron absorption is maximal in the duodenum unlike Ferrous Sulfate, which must be protected against oxidation through coating. The IntegraTM Capsule is superior to enteric coated or sustained release preparations that are apt to pass beyond the duodenum and would therefore be poorly absorbed. Ferrous Fumarate contrasts very favorably with the availability of the 20% of elemental iron of ferrous sulfate, and the 13% of elemental iron of ferrous gluconate. Calculation of Dose should be in terms of elemental iron and three doses ranging from 50-100 mg of elemental iron is usually adequate.
Polysaccharide Iron Complex: Provides about 62.5 mg elemental iron, as a cell-contracted akaganèite. It is a product of ferric iron complexed to a low molecular weight polysaccharide. This polysaccharide is produced by the extensive hydrolysis of starch and is a dark brown powder, that dissolves in water to form a very dark brown solution, which is virtually odorless and tasteless.
All IntegraTM products include a unique patented source of iron, e.g. Ferrous Fumarate and Polysaccharide Iron Complex (U.S. Patent No: 11/243,043 Pending). "An increase in tolerability is observed with the (patented formulation) and is believed to occur as the result of distributing the total iron content in the composition among compounds that provide iron to the patient's blood stream via two different mechanisms. The ferrous salts are readily absorbed in the upper gut, by direct dissolution and absorption of the ferrous iron by the bloodstream. However, the iron available from PIC is absorbed in the lower gut, via an active protein transport mechanism".
Clinical Studies: Because Ferrous Fumarate is an organic complex, it contains no free ions, either ferric or ferrous. Polysaccharide Iron Complex is clinically non-toxic. Prior studies in rats demonstrated that Polysaccharide Iron Complex (PIC), when administered as a single oral dose to Sprague Dawley rats, did not produce evidence of toxicity at a dosage level of 5000 mg Iron/kg: (An Acute Oral Toxicity Study in Rats with Polysaccharide-Iron Complex. T.N.Merriman, M. Aikman and R.E. Rush, Springborn Laboratories. Inc. Spencerville, Ohio Study No. 3340.1 March - April 1994). Other clinical studies had demonstrated that Poly-saccharide Iron gives a good hematopoietic response with an almost complete absence of the side effects usually associated with oral iron therapy. Picinni and Ricciotti suggested in 1982, that "the therapeutic effectiveness of Polysaccharide Iron Complex when compared with iron fumarate in the treatment of iron deficiency anemia, appears to be as active as the iron fumarate and as well tolerated, however, it exerted a greater influence on the level of hemoglobin and on the number of red cells..." and that, "it has been exceptionally well tolerated by all patients" (Picinni, L.-Ricciotti, M. 1982. Therapeutic effectiveness of an iron-polysaccharide complex in comparison with iron fumarate in the treatment of iron deficiency anemias): PANMINERVA MEDICA-EUROPA MEDICA, Vol. 24, No. 3, pp. 213-220 (July-September 1982). The patented source of iron used in IntegraTM (Ferrous Fumarate and Polysaccharide Iron Complex) provides a high level of elemental iron with a low incidence of gastric distress.
CONCLUSION: Based on the results of this study, the oral combination of Ferrous Fumarate and Polysaccharide Iron Complex was better tolerated and safer than the oral administration of Ferrous Fumarate alone. The conclusion of this research stated, that the addition of PIC to Ferrous Fumarate surprisingly allows the same concentration of Ferrous Fumarate to be better tolerated than the Ferrous Fumarate alone.
INDICATIONS: The treatment of iron deficiency anemia, which may occur due to an increased need for iron, a deficient intake of iron, or an excessive loss of iron.
CONTRAINDICATIONS: All iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis and hemolytic anemias.
WARNING: Accidental overdose is the leading cause of fatal poisoning in children under six. Keep this and all drugs out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS: Existing gastrointestinal diseases, i.e. peptic ulcers, regional enteritis, ulcerative colitis may be aggravated by causing the iron not to be absorbed and therefore, be ineffective in patients with steatorrhea or those with a partial gastrectomy. It is important to determine and treat the underlying cause of the anemia, in addition to the administration of IntegraTM. Indefinite administration of iron should be avoided.
DOSAGE AND ADMINISTRATION: Adults (persons over 12 years of age), One (1) capsule daily, between meals, or as prescribed by a physician. Do not exceed recommended dosage. Do not administer to children under the age of 12.
TIME AND DURATION: The hematologic response to orally administered iron is indicated by reticulocytosis of 2% to 10% depending on the severity of the anemia, beginning five to ten days after initiation of therapy. A rise in hemoglobin value is observable at the end of the second week. When therapy is adequate, hemoglobin value increases 0.1 to 0.2 gm per 100 ml of blood a day and usually becomes normal in two months. However, to improve body iron stores, treatment may be continued from 3 to 12 months, after the hemoglobin has returned to normal. If no satisfactory response is noted after three weeks of therapy, consideration should be given to (1) whether dosage recommendations have been followed, (2) blood loss is occurring simultaneously, (3) whether there are complicating causes, such as infection, defects in absorption or utilization, (4) whether diagnosis or iron-deficiency was correct.
WARNING: Do not administer to pediatric patients. Do not exceed recommended dosage. Keep this and all drugs out of reach of pediatric patients. If you are pregnant or nursing a baby, seek the advice of a health professional before using this product.
| TANDEM - F
ferrous fumarate and polysacchride iron complex and folic acid capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - US Pharmaceutical Corporation (048318224) |
| Registrant - US Pharmaceutical Corporation (048318224) |