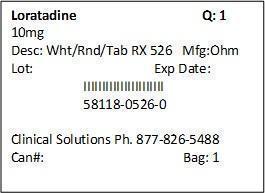
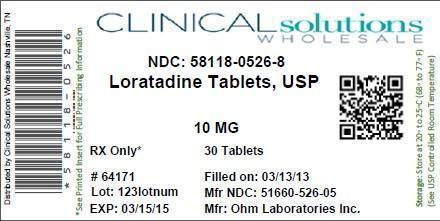
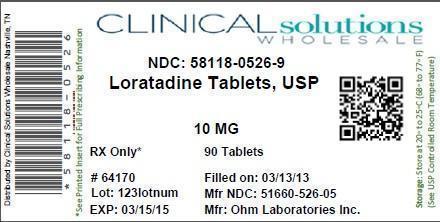
LORATADINE ALLERGY RELIEF- loratadine tablet
Clinical Solutions Wholesale
----------
Drug Facts
USE(S)
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
DIRECTIONS
|
adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver and kidney disease |
ask a doctor |
| LORATADINE ALLERGY RELIEF
loratadine tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Clinical Solutions Wholesale (078710347) |
| Registrant - Clinical Solutions Wholesale (078710347) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Clinical Solutions Wholesale | 078710347 | REPACK(58118-0526) , RELABEL(58118-0526) | |
Revised: 6/2017
Document Id: 72f05655-3a2a-4237-a95e-9f5a88a5e6d3
Set id: e1420f6f-8c08-4c4d-b44d-2d01f96ee123
Version: 2
Effective Time: 20170628
Clinical Solutions Wholesale